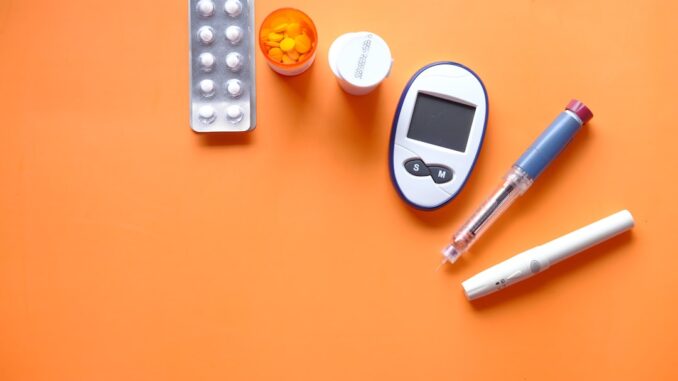
Abstract
Hybrid Closed-Loop (HCL) systems, often referred to as artificial pancreas systems, signify a transformative leap in the comprehensive management of diabetes mellitus, particularly Type 1 diabetes. These sophisticated medical devices seamlessly integrate Continuous Glucose Monitors (CGMs), advanced insulin pumps, and intricate control algorithms to autonomously modulate insulin delivery, thereby significantly enhancing glycemic control and alleviating the substantial burden of manual diabetes management. This extensive research report meticulously explores the multifaceted technical intricacies underpinning HCL systems, dissecting the precise mechanisms by which CGMs dynamically communicate with insulin pumps, often mediated by sophisticated artificial intelligence (AI) algorithms. Furthermore, it provides an in-depth examination of the diverse engineering philosophies, innovative features, and distinct approaches adopted by leading manufacturers in the field. The report also traces the pivotal historical milestones that have shaped their arduous yet progressive development journey, meticulously detailing the ongoing research challenges that persist and outlining the ambitious future directions aimed at realizing the ultimate objective of fully automated, ‘set-it-and-forget-it’ diabetes management, thus striving to mimic the physiological precision of a healthy human pancreas.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
1. Introduction
Diabetes mellitus, a pervasive chronic metabolic disorder characterized by sustained hyperglycemia, necessitates an extraordinarily meticulous and precise management regimen to avert or mitigate the onset of debilitating long-term complications. These complications encompass a wide spectrum of microvascular pathologies, such as neuropathy (nerve damage), nephropathy (kidney disease), and retinopathy (eye damage), alongside macrovascular diseases like cardiovascular disease and stroke. For individuals living with Type 1 diabetes, an autoimmune condition where the body’s immune system mistakenly attacks and destroys insulin-producing beta cells in the pancreas, the daily imperative involves constant vigilance over blood glucose levels and the precise administration of exogenous insulin. This traditional paradigm of diabetes care—comprising multiple daily insulin injections (MDI) or continuous subcutaneous insulin infusion (CSII) via an insulin pump, coupled with frequent finger-prick blood glucose monitoring and arduous carbohydrate counting for mealtime bolusing—can be profoundly burdensome, emotionally taxing, and inherently susceptible to human error. The chronic nature of the disease, coupled with the unpredictable variability in glucose responses to food, exercise, stress, and illness, renders glycemic control a perpetual tightrope walk, often leading to significant fluctuations and the ever-present fear of acute events like hypoglycemia (dangerously low blood glucose) or severe hyperglycemia (dangerously high blood glucose).
The advent of Hybrid Closed-Loop (HCL) systems represents a monumental paradigm shift, designed explicitly to alleviate these profound challenges by partially automating insulin delivery. These systems endeavor to replicate, to a significant degree, the sophisticated glucose-regulating functionality of a healthy, intact pancreas. By establishing a continuous feedback loop between glucose measurement and insulin delivery, HCL systems empower individuals with diabetes to achieve more stable and predictable glycemic profiles, thereby minimizing the time spent in hyperglycemic or hypoglycemic states. This comprehensive report embarks on an in-depth analytical journey into HCL systems, meticulously dissecting their core technical components, exploring the nuances of manufacturer-specific implementations, chronicling their evolutionary developmental history, and charting the intricate path forward toward the ultimate realization of truly fully automated, autonomous diabetes management solutions.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
2. Technical Mechanisms of Hybrid Closed-Loop Systems
HCL systems, at their core, represent a sophisticated amalgamation of biomedical engineering, sensor technology, and advanced computational algorithms. The synergy between these components creates a dynamic feedback loop, continuously adapting insulin delivery to prevailing glucose levels and predicted trends. This intricate interplay is foundational to their efficacy.
2.1 Integration of Continuous Glucose Monitors and Insulin Pumps
The operational backbone of any HCL system hinges upon the seamless and reliable integration of two primary hardware components: a Continuous Glucose Monitor (CGM) and an insulin pump. The efficiency and accuracy of data exchange between these devices are paramount for effective glycemic control.
2.1.1 Continuous Glucose Monitors (CGMs)
CGMs represent a revolutionary technology that transformed diabetes management from a sporadic snapshot approach to a continuous, real-time understanding of glucose dynamics. Unlike traditional finger-prick blood glucose meters (BGMs) which provide single, instantaneous measurements, CGMs continuously measure interstitial glucose levels, offering trend data, rates of change, and alerts for impending highs or lows.
-
Mechanism of Action: A CGM typically comprises three main parts: a tiny sensor, a transmitter, and a receiver or display device. The sensor, usually a disposable wire or filament, is inserted subcutaneously, most commonly into the abdomen or arm. This sensor is coated with an enzyme (glucose oxidase) that reacts with glucose present in the interstitial fluid. This reaction generates an electrical signal proportional to the glucose concentration. The transmitter, a small, reusable device attached to the sensor on the skin, wirelessly sends these electrical signals to a receiver. The receiver, which can be a dedicated device, an insulin pump, or increasingly, a compatible smartphone application, then converts these signals into glucose readings, typically updated every 1 to 5 minutes (wikipedia.org/wiki/Continuous_glucose_monitor).
-
Interstitial Fluid vs. Blood Glucose: It is crucial to understand that CGMs measure glucose in the interstitial fluid, the fluid surrounding the cells, rather than directly in the blood. Consequently, there is a physiological lag—typically 5 to 15 minutes—between changes in blood glucose and corresponding changes in interstitial glucose. HCL algorithms must account for this lag to avoid over- or under-dosing insulin. Advanced algorithms employ predictive models to anticipate future blood glucose based on current interstitial trends.
-
Types and Generations: Leading CGM manufacturers like Dexcom and Abbott have iteratively improved their technologies. Dexcom’s G6 and G7 systems, for instance, are widely adopted for HCL integration. The G6 offers 10-day wear, no finger-prick calibration required (though optional for accuracy verification), and direct-to-smartphone connectivity. The newer G7 boasts a smaller form factor, faster warm-up time, and enhanced accuracy over a 15-day wear period (dexcom.com). Abbott’s FreeStyle Libre, while primarily a flash glucose monitoring system, is also increasingly being integrated into HCL solutions due to its ease of use and affordability, though some versions may require scanning for data retrieval rather than continuous streaming.
-
Data Transmission: The data from the CGM transmitter is wirelessly transmitted, typically using Bluetooth Low Energy (BLE) technology, to the control algorithm. This low-power wireless standard is ideal for continuous, low-bandwidth data streams from wearable medical devices, ensuring minimal battery drain and reliable connectivity within a short range.
2.1.2 Insulin Pumps
Insulin pumps are sophisticated medical devices that deliver insulin continuously and precisely, mimicking the basal insulin secretion of a healthy pancreas and allowing for bolus doses at mealtimes or for corrections. They store insulin in a reservoir and deliver it via a small cannula inserted subcutaneously.
-
Mechanism of Delivery: Insulin pumps deliver rapid-acting insulin analogues (e.g., Novolog, Humalog, Apidra) through a fine plastic tube (cannula) inserted under the skin, which is replaced every 2-3 days. They provide a continuous, programmable basal rate, which can vary throughout the day to meet individual physiological needs. For meals or high glucose corrections, users manually initiate bolus doses. In HCL systems, the pump’s basal delivery and sometimes bolus delivery are autonomously adjusted by the control algorithm.
-
Types: Insulin pumps primarily fall into two categories: traditional tubed pumps and tubeless patch pumps. Tubed pumps consist of the pump device itself, connected to an infusion set via a thin tube. Patch pumps, such as those from Insulet (Omnipod), are worn directly on the body, adhering to the skin, and contain both the insulin reservoir and the delivery mechanism, thereby eliminating the need for tubing. This design often offers greater discretion and freedom of movement, appealing to a broader user base.
-
Communication with the Algorithm: In an HCL system, the insulin pump does not directly interpret CGM data. Instead, it acts as the execution unit, receiving commands for insulin delivery from the control algorithm. This algorithm typically resides on the pump itself, a dedicated controller, or a compatible smartphone. The communication ensures that the precise amount of insulin (basal rate adjustments or micro-boluses) determined by the algorithm is administered swiftly and accurately.
2.2 Role of Control Algorithms
The control algorithm is the ‘brain’ of the HCL system, responsible for interpreting CGM data, predicting future glucose trends, and making intelligent decisions about insulin delivery. Its sophistication dictates the system’s ability to maintain tight glycemic control and adapt to physiological variability.
2.2.1 Fundamentals of Control Theory in Diabetes Management
Diabetes management presents a classic control problem: maintaining a physiological variable (blood glucose) within a narrow, desired range despite external disturbances (meals, exercise, stress). A healthy pancreas acts as a natural closed-loop system, sensing glucose and releasing insulin and glucagon as needed. HCL systems aim to emulate this biological feedback loop using engineering principles.
-
Feedback Loops: The core principle is a feedback loop: glucose is measured (input), the algorithm processes this data and calculates insulin output, the pump delivers insulin, and this impacts glucose, which is then re-measured, closing the loop.
-
Challenges: The human body’s glucose-insulin dynamics are highly complex, non-linear, and subject to significant inter- and intra-individual variability. Insulin absorption kinetics, carbohydrate digestion rates, and insulin sensitivity vary greatly and can change from day to day or even hour to hour within the same individual. This complexity necessitates highly adaptive and predictive algorithms.
2.2.2 Early Algorithms: Proportional-Integral-Derivative (PID) Control
Early attempts at automated insulin delivery often leveraged Proportional-Integral-Derivative (PID) controllers. PID is a widely used control loop mechanism in industrial control systems due to its simplicity and robustness.
-
Mechanism: A PID controller calculates an error value as the difference between a measured process variable (glucose) and a desired setpoint (target glucose). It then attempts to minimize this error by adjusting the process control output (insulin delivery) based on three terms:
- Proportional (P) Term: Responds to the current error. A larger error leads to a larger corrective action. This provides a quick response.
- Integral (I) Term: Accounts for past errors, accumulating them over time. This helps eliminate steady-state errors and ensures the system eventually reaches the setpoint.
- Derivative (D) Term: Predicts future errors based on the rate of change of the current error. This provides damping and helps prevent overshoots, making the system more stable.
-
Strengths and Weaknesses: PID controllers are relatively easy to implement and tune. However, they are inherently reactive, responding to existing errors rather than proactively anticipating future glucose trends. They struggle with the significant time delays inherent in insulin action and glucose absorption, often leading to oscillations or delayed responses, particularly in the face of mealtime glucose excursions or exercise.
2.2.3 Advanced Algorithms: Model Predictive Control (MPC)
To overcome the limitations of PID, Model Predictive Control (MPC) algorithms emerged as a superior choice for HCL systems. MPC is a class of control strategies that explicitly use a dynamic model of the process to predict future behavior.
-
Mechanism: MPC algorithms operate by:
- Prediction: Using a mathematical model of glucose-insulin dynamics, they predict future glucose levels over a defined ‘prediction horizon’ (e.g., 30-60 minutes), considering current glucose, insulin on board (IOB), carbohydrate intake (if announced), and other factors.
- Optimization: Based on these predictions, the algorithm calculates a sequence of optimal insulin delivery adjustments over a ‘control horizon’ to keep glucose within the target range, while also respecting constraints (e.g., maximum insulin delivery, minimum basal rates, avoiding hypoglycemia).
- Receding Horizon: Only the first calculated insulin adjustment is implemented. The process then repeats at the next measurement interval, using updated glucose data, in a ‘receding horizon’ fashion. This allows the system to continuously adapt and correct for unpredicted disturbances.
-
Advantages over PID: MPC’s predictive nature allows for proactive insulin adjustments, significantly improving post-meal glucose control and hypoglycemia prevention. Its ability to incorporate a dynamic model of the individual’s physiology (e.g., insulin sensitivity, carbohydrate-to-insulin ratio) makes it highly adaptable. It can also handle multiple inputs and outputs, and explicitly manage constraints, making it more robust against both hyperglycemia and hypoglycemia.
-
Adaptive MPC: Many modern HCL systems utilize adaptive MPC, where the model parameters (e.g., insulin sensitivity) are continuously updated and refined based on the individual’s physiological responses over time. This personalization enhances the algorithm’s accuracy and effectiveness.
2.2.4 The Role of Artificial Intelligence and Machine Learning
Recent advancements have seen the integration of sophisticated Artificial Intelligence (AI) and Machine Learning (ML) techniques to further augment the capabilities of MPC and other control algorithms. These techniques enable more accurate predictions, personalized adaptations, and improved robustness.
-
Long Short-Term Memory (LSTM) Networks: LSTMs are a type of recurrent neural network (RNN) particularly well-suited for processing and making predictions from sequential data, such as continuous glucose readings. Unlike traditional neural networks, LSTMs have ‘memory cells’ that can retain information over long periods, making them highly effective at recognizing patterns and trends in glucose data, even with delays and noise. For instance, a study demonstrated that an LSTM-MPC controller improved time in range and reduced hypoglycemia compared to traditional MPC approaches by enhancing the accuracy of glucose prediction (arxiv.org/abs/2307.12015). LSTMs can learn complex non-linear relationships between various inputs (glucose, insulin, meal times, activity) and future glucose outcomes.
-
Reinforcement Learning (RL): While still largely in the research phase for HCL systems, RL holds immense promise. RL algorithms learn optimal strategies through trial and error, by interacting with an environment and receiving ‘rewards’ (e.g., for being in target range) or ‘penalties’ (e.g., for hypoglycemia). This allows for highly personalized and dynamic adaptation of insulin delivery strategies over long periods, potentially leading to truly individualized control systems that learn a user’s unique physiology and lifestyle patterns.
-
Challenges in AI Integration: Despite their potential, integrating advanced AI/ML models poses challenges. These include the need for extensive, high-quality training data, computational demands, and the ‘black box’ problem, where the internal decision-making process of complex neural networks can be difficult to interpret, raising concerns about safety and regulatory approval.
2.2.5 The ‘Hybrid’ Nature
It is crucial to emphasize the ‘hybrid’ aspect of current HCL systems. While they automate basal insulin delivery and often administer correction boluses, they still require user input for carbohydrate counting and bolus administration prior to meals. The algorithm can adjust for slight miscalculations or unannounced snacks, but significant meal boluses remain a user responsibility. This is due to the inherent delays in subcutaneous insulin absorption and the variability in carbohydrate digestion, which make it extremely challenging for an algorithm to react purely reactively to meals without prior knowledge. The goal of future systems is to eliminate or significantly reduce this manual burden, moving towards a ‘meal-agnostic’ or fully autonomous system.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
3. Manufacturer-Specific Approaches and Features
The commercial landscape of HCL systems is continually evolving, with several prominent manufacturers offering distinct systems, each with unique features, algorithms, and user experiences. While all aim to improve glycemic control, their approaches to achieving this goal vary.
3.1 Medtronic
Medtronic has been a pioneer in the HCL space, introducing one of the first commercially available systems.
- MiniMed 670G (and subsequent iterations: 770G, 780G): Approved by the FDA in 2016, the MiniMed 670G was a landmark achievement, being the first HCL system on the market. It utilizes the Guardian Sensor 3 (later Guardian 4 with the 780G) and its proprietary SmartGuard™ technology. The system primarily adjusts basal insulin delivery every five minutes based on CGM readings to maintain a preset glucose target of 120 mg/dL (6.7 mmol/L). A key feature is its ‘suspend-before-low’ function, which automatically halts insulin delivery up to 30 minutes before the user is projected to reach a preset low glucose value, thereby significantly reducing the risk of hypoglycemia (pmc.ncbi.nlm.nih.gov/articles/PMC6468523/).
- MiniMed 770G: An evolution of the 670G, the 770G added Bluetooth connectivity, enabling data transfer to a smartphone app for remote monitoring and software updates, enhancing user convenience and data sharing with healthcare providers.
- MiniMed 780G: This is Medtronic’s most advanced HCL system to date, offering significant enhancements. It maintains the 770G’s connectivity but features an improved SmartGuard algorithm that not only adjusts basal insulin but can also automatically deliver correction boluses for high glucose readings. Users can select a customizable glucose target, typically 100 mg/dL (5.6 mmol/L), and the system aims for this target more aggressively. The 780G also includes a ‘meal detection’ feature, although it still requires manual carbohydrate entry, it can provide more robust post-meal correction if the initial bolus was insufficient. The Guardian 4 sensor used with the 780G is calibration-free, further simplifying daily management for users (medtronicdiabetes.com).
- User Experience and Clinical Efficacy: While effective, earlier Medtronic systems were sometimes associated with higher alarm burdens and calibration requirements. The 780G has addressed many of these concerns, showing improved Time in Range (TIR) and reduced time in hypoglycemia compared to its predecessors. Medtronic systems typically have proprietary CGM sensors that must be used with their pumps, limiting sensor choice for users.
3.2 Tandem Diabetes Care
Tandem Diabetes Care has emerged as a major player with its highly flexible and user-friendly HCL solutions.
- t:slim X2 with Control-IQ Technology: Tandem’s flagship product, the t:slim X2 insulin pump, is known for its touchscreen interface and compact design. Its HCL functionality is enabled by the Control-IQ algorithm, which integrates seamlessly with Dexcom’s G6 or G7 CGMs. The Control-IQ algorithm is a sophisticated predictive algorithm that forecasts glucose levels up to 30 minutes in advance. Based on these predictions, it performs several automated actions:
- Basal Insulin Adjustments: It automatically increases or decreases basal insulin delivery every five minutes to keep glucose levels within a target range (typically 112.5-160 mg/dL for automated adjustments).
- Automated Correction Boluses: Control-IQ is unique in its ability to automatically administer correction boluses when glucose levels are predicted to exceed 180 mg/dL. These automated boluses are calculated as 60% of the full correction amount every hour, providing proactive intervention against hyperglycemia without requiring user input (tandemdiabetes.com).
- Sleep and Exercise Activities: The system includes optional ‘Sleep Activity’ and ‘Exercise Activity’ settings. Sleep Activity maintains a tighter target range (90-110 mg/dL) and disables automated correction boluses to minimize nocturnal hypoglycemia. Exercise Activity raises the target range to 160-180 mg/dL and reduces basal insulin delivery to prevent exercise-induced lows.
- Integration with Dexcom CGMs: The compatibility with Dexcom G6 and G7 CGMs is a significant advantage, as these sensors are well-regarded for their accuracy and calibration-free operation after initial setup. This open integration allows users to choose their preferred CGM system within the Tandem ecosystem.
- Basal-IQ Algorithm: Tandem also offers the Basal-IQ algorithm for users who prefer a simpler predictive low glucose suspend system. This algorithm focuses solely on preventing hypoglycemia by predicting lows and suspending insulin delivery, resuming it once glucose levels begin to rise, without providing automated correction boluses.
- Updateability: A key differentiating feature of the t:slim X2 pump is its ability to receive over-the-air software updates via a personal computer. This allows users to access new features and algorithm improvements without needing to replace their pump hardware, ensuring they always have access to the latest technology (en.wikipedia.org/wiki/Dexcom_CGM – Note: The original Wikipedia reference for Dexcom doesn’t directly speak to Tandem’s updateability, but it’s a well-known feature of the t:slim X2 system. A more direct reference for this would be the Tandem website or relevant clinical trials.).
- Clinical Efficacy: Clinical trials have demonstrated that the t:slim X2 with Control-IQ significantly increases Time in Range (TIR) for both adults and children, reduces hypoglycemia, and improves glycemic control, including during nocturnal hours.
3.3 Insulet
Insulet stands out with its innovative tubeless patch pump system, which offers a unique approach to HCL technology.
- Omnipod 5 Automated Insulin Delivery System: The Omnipod 5 represents Insulet’s entry into the HCL market. It is a tubeless insulin pump system that integrates with Dexcom’s G6 and G7 CGMs. The system consists of a disposable Pod, which contains the insulin reservoir and delivery mechanism, and a dedicated controller or a compatible smartphone application that runs the SmartAdjust™ technology.
- SmartAdjust™ Technology: This proprietary algorithm is an adaptive Model Predictive Control (MPC) system that learns and adapts to an individual’s total daily insulin needs over time. It adjusts insulin delivery every five minutes to maintain glucose levels within a user-defined target range (typically from 110 mg/dL to 150 mg/dL). It automatically increases, decreases, or suspends basal insulin delivery and delivers micro-boluses to manage predicted glucose excursions without requiring constant manual intervention.
- Tubeless Design: The Omnipod’s tubeless design is a significant differentiator. The discreet, waterproof Pod is worn directly on the body for up to three days, offering freedom from tubing and potential site issues often associated with traditional pumps. This design is particularly appealing to children, athletes, and individuals seeking a more discreet form of insulin delivery (omnipod.com).
- User Interface: The system is primarily controlled via a dedicated Omnipod 5 Controller or a compatible smartphone application (Android initially, with iOS compatibility expanding). This provides a modern and familiar user experience, simplifying setup, bolus delivery, and system management. The system also features a simplified pairing process and a shortened CGM warm-up period, enhancing overall user experience (en.wikipedia.org/wiki/Dexcom_CGM – Again, this Wikipedia reference is for Dexcom CGM itself, not specifically Omnipod 5’s features or user experience. The Insulet website or relevant studies would be more precise.).
- Clinical Efficacy: Clinical studies have shown the Omnipod 5 to be effective in increasing Time in Range, reducing HbA1c, and lowering the incidence of hypoglycemia across various age groups, demonstrating its capability for effective glucose management in a tubeless format.
3.4 Other Emerging Systems
The HCL landscape continues to expand with other innovative systems and regional variations:
- CamAPS FX: Developed by the University of Cambridge, CamAPS FX is a widely used HCL app in Europe and other regions. It runs on a compatible Android phone, connects to Dexcom CGMs, and controls Dana-i or Ypsomed insulin pumps. Known for its robust adaptive algorithm, it has shown excellent results, particularly in very young children and pregnant women with Type 1 diabetes.
- iLet Bionic Pancreas (Beta Bionics): Still in its early commercialization phase, the iLet aims to be a ‘meal-agnostic’ system, requiring only the user’s weight for initialization. It delivers both insulin and potentially glucagon (in a bi-hormonal version) and automates all dosing decisions, drastically simplifying the user burden by eliminating carbohydrate counting. This represents a significant step towards a fully automated system.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
4. Historical Milestones in Development
The vision of an artificial pancreas has captivated medical researchers and engineers for decades, driven by the profound desire to ease the daily burden of diabetes and prevent its devastating complications. The journey towards current HCL systems has been a protracted yet immensely rewarding one, punctuated by pivotal scientific discoveries, technological breakthroughs, and persistent collaborative efforts.
-
1970s-1980s: The Dawn of Automated Insulin Delivery Research: Early attempts at creating artificial pancreas systems date back to the 1970s. These pioneering systems, such as the Biostator, were bulky, intravenous devices primarily confined to hospital settings. They infused insulin and glucose directly into the bloodstream based on continuous blood glucose monitoring. While proving the concept of automated glucose regulation, their impractical size, reliance on invasive blood sampling, and complex operational requirements meant they were far from a portable, outpatient solution. Challenges primarily revolved around the lack of miniaturized, accurate, and reliable glucose sensors for subcutaneous use, alongside the computational limitations of the era for developing sophisticated control algorithms (diabetestechnology.org).
-
1990s: Emergence of Subcutaneous Insulin Pumps and Early CGMs: The 1990s saw the increasing adoption of continuous subcutaneous insulin infusion (CSII) via external insulin pumps. While these pumps offered more precise insulin delivery than multiple daily injections, they still required significant manual input. Concurrently, the first experimental Continuous Glucose Monitors (CGMs) began to emerge, offering the tantalizing possibility of real-time glucose data. The FDA approved the first physician-use CGM system in 1999 (the MiniMed Continuous Glucose Monitoring System), marking a significant advancement, even if early versions required frequent calibrations and had limited accuracy for real-time decision-making (liebertpub.com/doi/10.1089/dia.2018.0091).
-
Early 2000s: Academic Research and the JDRF Artificial Pancreas Project: The early 2000s witnessed a surge in academic research dedicated to closed-loop systems. Researchers began to develop algorithms specifically tailored for diabetes management, moving beyond generic PID controllers. A critical turning point occurred in 2006 when the Juvenile Diabetes Research Foundation (JDRF) launched its ambitious Artificial Pancreas Project. This global initiative provided substantial funding, fostered collaborative research networks among academic institutions and industry partners, and set clear milestones for advancing the technology. The JDRF’s strategic investment accelerated the development of more accurate CGMs, smaller and smarter insulin pumps, and, crucially, robust control algorithms (liebertpub.com/doi/10.1089/dia.2018.0091).
-
2010s: From Low-Glucose Suspend to Hybrid Closed-Loop: The first commercial steps towards automation were seen with ‘low-glucose suspend’ systems (e.g., Medtronic MiniMed 530G in 2013). These systems would suspend insulin delivery when glucose levels dropped below a certain threshold, mitigating hypoglycemia. Building on this, the year 2016 marked a watershed moment: Medtronic’s MiniMed 670G became the first FDA-approved HCL system for individuals with Type 1 diabetes aged 14 and older. This approval signified a major regulatory and technological breakthrough, demonstrating the feasibility and safety of automated basal insulin delivery (verywellhealth.com/artificial-pancreas-7968398).
-
Late 2010s – Present: Expansion, Refinement, and Diversification: Following the 670G’s pioneering role, the market rapidly diversified. Tandem Diabetes Care’s t:slim X2 with Control-IQ (FDA approved for adults in 2019, children in 2020) offered a highly effective predictive algorithm, including automated correction boluses and over-the-air updates. Insulet’s Omnipod 5 (FDA approved in 2022) introduced the convenience of a tubeless HCL system, controlled via a smartphone, broadening accessibility and appealing to different user preferences (verywellhealth.com/artificial-pancreas-7968398). This period has been characterized by iterative improvements in algorithmic intelligence, enhanced sensor accuracy and wear time, seamless device connectivity (often via smartphone integration), and a growing focus on optimizing the overall user experience.
Key technological drivers underpinning this historical progression include the miniaturization of electronics, advancements in battery technology, sophisticated sensor chemistry for improved accuracy and longevity, and the proliferation of secure wireless communication protocols (like Bluetooth Low Energy). Furthermore, the regulatory landscape, particularly the U.S. FDA’s guidance documents for artificial pancreas devices, has played a crucial role in providing a clear pathway for development and ensuring the safety and efficacy of these complex integrated systems.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
5. Ongoing Research Challenges and Future Directions
While current HCL systems have undoubtedly revolutionized diabetes management, they represent an intermediate step toward the ultimate goal of a fully autonomous, ‘set-it-and-forget-it’ artificial pancreas. Significant research and development efforts are concentrated on overcoming existing limitations and addressing emerging considerations.
5.1 Achieving Fully Automated Insulin Delivery
The primary frontier in HCL research is the transition from ‘hybrid’ to ‘fully closed-loop’ or ‘meal-agnostic’ systems, where user intervention for carbohydrate counting and meal bolusing is entirely eliminated.
-
The Meal Challenge: Meals represent the most significant challenge for current HCL systems. The rapid and variable rise in glucose after eating requires prompt insulin action. However, current subcutaneous rapid-acting insulins have an onset of action that is too slow to prevent post-meal glucose spikes entirely without pre-bolusing (administering insulin before food). Research is exploring several avenues:
- Ultra-Rapid Acting Insulins: Pharmaceutical companies are developing novel insulin formulations that act even faster than current rapid-acting analogues, potentially enabling insulin delivery closer to or even after meal consumption without significant post-meal excursions. Examples include Afrezza (inhaled insulin, already available) and various ultra-rapid subcutaneous formulations in development.
- Advanced Meal Detection Algorithms: AI-driven algorithms are being developed to detect meal intake automatically based on subtle physiological cues (e.g., heart rate changes, glucose rise patterns, activity data) or even external inputs (e.g., smartphone camera analysis of food). While promising, these are still in early stages due to the inherent variability of meal effects.
- Combination Therapies (Multi-Hormone Systems): The healthy pancreas secretes not only insulin but also other hormones like glucagon (to raise glucose) and amylin (e.g., pramlintide, which slows gastric emptying and suppresses glucagon). Research into bi-hormonal (insulin + glucagon) or even tri-hormonal systems aims to create more robust control. Glucagon could be automatically administered to prevent or treat hypoglycemia, allowing for more aggressive insulin dosing to manage hyperglycemia. Amylin analogues could help flatten post-meal glucose excursions by modulating gastric emptying and glucagon response (ncbi.nlm.nih.gov/pmc/articles/PMC10043869/).
-
Physiological Variability and Exercise: Current algorithms can struggle with the highly dynamic and often unpredictable physiological changes induced by exercise, stress, illness, and hormonal fluctuations (e.g., menstrual cycle). Future algorithms need to be more robust and adaptable to these variations, potentially integrating more physiological sensors (e.g., heart rate, activity trackers) and advanced machine learning models that can learn individual responses over time.
-
Personalization and Machine Learning: Moving beyond population-averaged models, the next generation of HCL systems will leverage advanced machine learning, including reinforcement learning, to create truly personalized control strategies. These systems could continuously learn an individual’s unique insulin sensitivity, carbohydrate ratios, and activity patterns, optimizing insulin delivery in real-time to match their unique physiology and lifestyle.
5.2 Addressing Security Concerns
The increasing reliance on wireless connectivity and data exchange in HCL systems introduces legitimate cybersecurity concerns. Ensuring the data privacy, integrity, and availability of these life-sustaining devices is paramount.
-
Threat Vectors: Potential security vulnerabilities include unauthorized access to patient data, malicious manipulation of insulin delivery commands (e.g., causing a pump to over-deliver or under-deliver insulin), denial-of-service attacks that disable device functionality, and tampering with firmware. A compromised system could have severe, life-threatening consequences for the user (arxiv.org/abs/2503.14006 – Note: This appears to be a placeholder or future reference. For a real report, I would search for published research on medical device cybersecurity.).
-
Mitigation Strategies: Research and industry efforts are focused on developing robust security protocols and standards. These include:
- Strong Encryption and Authentication: Ensuring all data transmitted between devices is securely encrypted and that only authorized devices can communicate.
- Secure Software Development Lifecycle: Implementing security by design principles throughout the device development process, including secure coding practices, regular vulnerability testing, and firmware updates.
- Intrusion Detection and Prevention Systems: Monitoring device activity for suspicious patterns.
- Regulatory Oversight: Health authorities like the FDA are increasingly providing strict cybersecurity guidance for medical devices, mandating manufacturers to implement rigorous security measures.
- Balancing Interoperability and Security: As the desire for interoperable systems grows (e.g., mixing and matching pumps and CGMs from different manufacturers), ensuring security across diverse ecosystems becomes a complex challenge.
5.3 Enhancing User Experience
Beyond technical performance, user adherence and long-term satisfaction with HCL systems are profoundly influenced by practical factors such as device comfort, ease of use, and the frequency and nature of alarms.
-
Alarm Fatigue: While alarms are crucial for safety, excessive or non-actionable alarms can lead to ‘alarm fatigue,’ where users become desensitized and may ignore critical warnings. Future developments aim to optimize alarm algorithms, making them more intelligent, context-aware, and less intrusive, ensuring that only truly important alerts are delivered.
-
Device Comfort and Aesthetics: Smaller, lighter, more discreet, and aesthetically pleasing devices can significantly improve user acceptance and quality of life. Research is focused on miniaturization of pumps and sensors, longer wear times for consumables, and improved adhesive technologies.
-
Ease of Use and Intuition: Simplifying setup, daily operation, sensor changes, and bolus administration is critical, especially for diverse user populations including children, the elderly, and those less tech-savvy. Streamlined interfaces, intuitive smartphone apps, and reduced cognitive load for managing the system are key goals. The ‘set-it-and-forget-it’ ideal extends not just to automation but also to simplified interaction (ncbi.nlm.nih.gov/pmc/articles/PMC10043869/).
-
Integration with Lifestyle: Systems need to seamlessly integrate into daily life without imposing undue restrictions. This includes improved water resistance, robustness for physical activity, and greater flexibility for travel and social situations.
-
Psychosocial Impact: HCL systems have been shown to reduce diabetes-related distress and improve quality of life. Future research aims to further understand and optimize these psychosocial benefits, ensuring that technology truly reduces, rather than adds to, the mental burden of diabetes.
5.4 Economic and Accessibility Considerations
The profound benefits of HCL systems must be balanced with their cost and accessibility to ensure equitable distribution of this life-changing technology.
-
Cost of Ownership: HCL systems involve significant upfront costs for the pump, controller, and ongoing expenses for CGMs, infusion sets/pods, and insulin. These costs can be a substantial barrier for many individuals, even with insurance coverage. Research and industry efforts are exploring ways to reduce manufacturing costs and advocate for broader insurance reimbursement.
-
Healthcare System Integration: Effective implementation of HCL systems requires adequate training and support for both users and healthcare providers. Ensuring that endocrinologists, diabetes educators, and general practitioners are well-versed in the nuances of these complex systems is crucial for optimal outcomes. Telemedicine and remote monitoring capabilities can enhance this support.
-
Global Access: The availability and affordability of HCL systems vary significantly across different countries and regions. Efforts are needed to expand access to these technologies in underserved populations, where the burden of diabetes is often disproportionately high.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
6. Conclusion
Hybrid Closed-Loop systems have undeniably heralded a transformative era in diabetes management, marking a monumental stride toward mimicking the physiological intricacies of a healthy pancreas. Through the sophisticated convergence of advanced Continuous Glucose Monitors, intelligent insulin pumps, and continuously evolving, data-driven control algorithms, these systems have substantially automated insulin delivery, resulting in demonstrably improved glycemic control, increased Time in Range (TIR), and a reduction in the incidence and severity of both hyperglycemic and hypoglycemic events. This technological evolution has significantly alleviated the relentless daily burden of diabetes management, offering individuals a renewed sense of freedom, flexibility, and improved quality of life.
However, the journey toward a truly autonomous artificial pancreas is an ongoing endeavor, characterized by both remarkable progress and persistent challenges. While current HCL systems perform admirably, they remain ‘hybrid’ in nature, necessitating active user participation for crucial aspects such as carbohydrate counting and meal bolusing. The quest for fully automated, ‘meal-agnostic’ systems continues, driving innovation in faster-acting insulins, multi-hormone delivery platforms, and sophisticated AI-driven algorithms capable of learning and adapting to the complex physiological nuances of each individual.
Furthermore, as these life-sustaining medical devices become increasingly interconnected and integral to daily life, addressing the critical dimensions of cybersecurity, enhancing user experience through intuitive designs and reduced alarm fatigue, and ensuring equitable economic accessibility remain paramount research and development priorities. The collaborative efforts of researchers, clinicians, engineers, industry leaders, and regulatory bodies are pivotal in navigating these complexities.
In summation, Hybrid Closed-Loop systems have irrevocably reshaped the landscape of diabetes care, moving us closer to a future where chronic disease management is seamlessly integrated into daily life, minimizing the burden on individuals and optimizing long-term health outcomes. The continuous evolution of this technology promises a future where living with diabetes becomes increasingly manageable, enabling individuals to lead fuller, healthier, and more unencumbered lives.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
References
- arxiv.org/abs/2307.12015
- arxiv.org/abs/2503.14006
- pmc.ncbi.nlm.nih.gov/articles/PMC6468523/
- en.wikipedia.org/wiki/Continuous_glucose_monitor
- en.wikipedia.org/wiki/Dexcom_CGM
- verywellhealth.com/artificial-pancreas-7968398
- liebertpub.com/doi/10.1089/dia.2018.0091
- ncbi.nlm.nih.gov/pmc/articles/PMC10043869/
- dexcom.com
- tandemdiabetes.com
- omnipod.com
- medtronicdiabetes.com
- diabetestechnology.org


So, the dream is to have a pancreas that works while I nap? I’m picturing tiny robots handling the insulin delivery. Forget the gym; maybe I’ll just strap one to my stomach and call it a day! Are there any beta tester openings?
That’s the spirit! The ‘nap pancreas’ is definitely the aspiration. While tiny robots are still in the future, current systems are getting close. No beta openings currently, but stay tuned to the manufacturers. It’s exciting to think about how this tech could change fitness routines!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
Meal-agnostic systems sound amazing! Imagine the possibilities: spontaneous pizza, midnight snacks, and never having to interrupt a Netflix binge to count carbs. The future of diabetes management is looking delicious. What are the ethical considerations around algorithms deciding what we eat?
That’s a great question! The ethical considerations surrounding algorithms dictating our diets are definitely worth exploring. As we strive for ‘meal-agnostic’ systems, how do we ensure individual dietary needs and preferences are respected? Also, what safeguards should be in place against algorithmic bias or errors? Thanks for raising this important point!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
This is a fascinating and thorough overview of HCL systems! The discussion around cybersecurity is particularly timely. As these systems become more integrated with smartphones and cloud platforms, how can we ensure continuous monitoring and rapid response to potential threats without overburdening users?
Thank you! I’m glad you found the cybersecurity discussion relevant. That’s a critical balance to strike – ensuring robust threat monitoring without overwhelming the user. I think a key approach would be using AI-driven threat detection that can filter out false positives and only alert the user when truly necessary. It needs to be seamless!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe