Understanding RuBisCO: The Earth’s Most Abundant Enzyme and the Quest for Photosynthetic Enhancement
Many thanks to our sponsor Esdebe who helped us prepare this research report.
Abstract
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) stands as the quintessential enzyme of life on Earth, unparalleled in its abundance and pivotal role in sustaining global ecosystems. It serves as the primary catalyst for the entry of atmospheric carbon dioxide into the biosphere, initiating the vast majority of primary productivity through the fixation of inorganic carbon into organic molecules. This enzymatic action underpins photosynthesis, the fundamental process by which plants, algae, and cyanobacteria convert light energy into chemical energy, ultimately forming the base of nearly all food webs. Despite its central and indispensable role in the global carbon cycle, RuBisCO is paradoxically characterized by significant inherent inefficiencies. These include a remarkably low catalytic turnover rate, meaning it processes substrates slowly compared to most enzymes, and a problematic dual substrate specificity that allows it to react with oxygen (O₂) in addition to its intended substrate, carbon dioxide (CO₂). The oxygenation reaction leads to a wasteful process known as photorespiration, which consumes energy, releases fixed carbon, and significantly diminishes the overall efficiency of photosynthesis. This comprehensive report delves into the intricate structure, diverse functions, profound evolutionary history, and the formidable challenges encountered in ambitious efforts to engineer this ubiquitous enzyme for enhanced efficiency in photosynthetic organisms. A particular focus is placed on the profound implications of such advancements for agricultural productivity and global food security in an era of changing climatic conditions.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
1. Introduction: The Cornerstone of Carbon Assimilation
RuBisCO, or Ribulose-1,5-bisphosphate carboxylase/oxygenase, is far more than just another enzyme; it is the linchpin of life as we know it, orchestrating the initial steps of carbon assimilation for nearly all autotrophic organisms. Its name succinctly describes its function: it catalyzes the carboxylation of ribulose-1,5-bisphosphate (RuBP), an event that serves as the entry point for atmospheric carbon into the biological world. This reaction is the defining step of the Calvin-Benson-Bassham (CBB) cycle, an intricate metabolic pathway that culminates in the synthesis of sugars and other organic compounds essential for growth and energy storage within photosynthetic organisms. The enzyme’s staggering abundance – estimated to comprise up to 50% of the total soluble protein in green leaves, making it the most abundant protein on the planet – unequivocally underscores its unparalleled importance in global biogeochemical cycles. Annually, RuBisCO is responsible for fixing an estimated 100 to 200 billion tonnes of carbon from the atmosphere, a colossal contribution that dwarfs all other carbon fixation processes combined and directly supports the vast majority of biomass production globally (The Science Notes, n.d.).
Its ubiquitous presence across the phylogenetic spectrum of photosynthetic life forms, from ancient cyanobacteria and unicellular algae to complex higher plants, highlights its fundamental and irreplaceable role. Despite this indisputable centrality, RuBisCO possesses inherent inefficiencies that have long fascinated and frustrated scientists. Its relatively sluggish catalytic rate (k_cat), which is orders of magnitude slower than that of many other enzymes, limits the overall pace of photosynthesis. Even more problematic is its propensity to engage in a competing reaction with molecular oxygen, leading to the initiation of the photorespiratory pathway. This metabolic shunt acts as a significant drain on photosynthetic efficiency, consuming valuable energy (ATP and NADPH) and resulting in the re-release of previously fixed carbon dioxide. These inefficiencies represent a major bottleneck to photosynthetic productivity, particularly in agricultural systems where the demand for increased crop yields is ever-growing amidst a rapidly expanding global population and the challenges of climate change. Consequently, understanding RuBisCO’s intricate mechanisms, its evolutionary journey, and the formidable challenges in engineering its function has become a paramount goal in molecular biology, biochemistry, and plant biotechnology, driving extensive research aimed at unlocking its full potential for a more productive and sustainable future.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
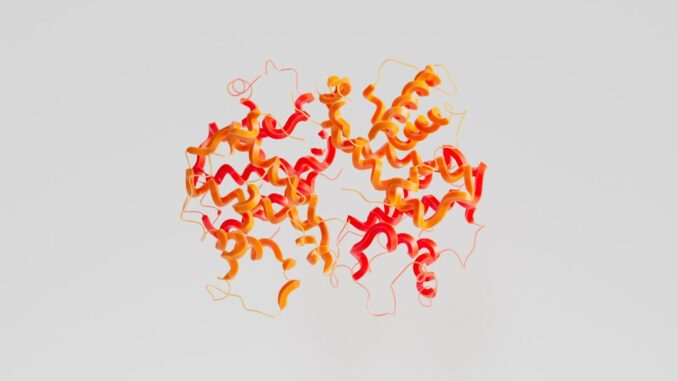
2. Structure of RuBisCO: A Symphony of Subunits and Domains
RuBisCO is a highly complex enzyme, displaying remarkable structural diversity across different biological kingdoms, yet retaining core functional similarities. Its architecture is typically multimeric, composed of large and, in most cases, small subunits that assemble into intricate quaternary structures crucial for its catalytic activity and stability.
2.1. Forms of RuBisCO
Evolutionary pressures and diverse environmental niches have led to the diversification of RuBisCO into several distinct forms, categorized primarily by their quaternary structure and phylogenetic relationships:
- Form I RuBisCO: This is the most prevalent and well-studied form, characteristic of plants, green algae, red algae, and cyanobacteria. It is a hexadecameric enzyme, meaning it is composed of 16 polypeptide chains. Specifically, it consists of eight large subunits (RbcL, approximately 55 kDa each) and eight small subunits (RbcS, typically 12-18 kDa each), forming an L8S8 complex. The large subunits are arranged in two stacked tetramers (L4L4), with the small subunits capping the top and bottom of this central large subunit core. Form I RuBisCO contains eight catalytic sites, one within each large subunit (RuBisCO, n.d.).
- Form II RuBisCO: Found in certain proteobacteria (e.g., Rhodospirillum rubrum) and some dinoflagellates, Form II enzymes are simpler in structure. They typically exist as dimers of large subunits (L2) or sometimes as hexamers (L6), lacking the small subunit entirely. While still catalyzing both carboxylation and oxygenation, Form II RuBisCO generally exhibits lower catalytic rates and a lower specificity for CO₂ over O₂ compared to Form I enzymes (The Science Notes, n.d.).
- Form III RuBisCO: Predominantly found in archaea and some anaerobic bacteria, Form III RuBisCOs are typically decamers or dodecamers of large subunits (L10 or L12). These forms are often associated with carbon fixation pathways in anaerobic environments, sometimes utilizing different substrates or operating within metabolic contexts distinct from oxygenic photosynthesis. They are generally considered ancient forms, reflecting early evolutionary trajectories in an anoxic world.
- Form IV RuBisCO (RuBisCO-like proteins): This category comprises proteins that share sequence homology with RuBisCO large subunits but lack conserved active site residues and catalytic activity. They are not involved in carbon fixation but rather in other metabolic pathways, such as the methionine salvage pathway, suggesting an evolutionary divergence from the core CO₂-fixing function. Their existence highlights the evolutionary plasticity of the RuBisCO scaffold.
2.2. Subunit Architecture and Function
2.2.1. Large Subunit (RbcL)
The RbcL subunit is the heart of RuBisCO’s catalytic machinery. In Form I enzymes, each large subunit is approximately 55 kDa and contains the complete catalytic site. Structurally, RbcL is typically composed of two distinct domains:
- N-terminal domain: This domain is largely involved in inter-subunit contacts, playing a role in the precise assembly of the multimeric enzyme.
- C-terminal domain: This domain forms a characteristic eight-stranded α/β-barrel structure (often called a TIM barrel), which houses the active site. Within this barrel, residues from both the N-terminal and C-terminal domains contribute to forming the substrate-binding pocket and catalytic machinery. Key residues within this domain are responsible for binding RuBP and the CO₂ or O₂ substrate.
Crucially, each large subunit contains a specific lysine residue (Lys201 in spinach RuBisCO, Lys201 in tobacco RuBisCO, but position varies slightly between species) that undergoes a reversible post-translational modification known as carbamylation. This carbamylation involves the binding of a CO₂ molecule, distinct from the substrate CO₂, to the ε-amino group of this lysine residue, forming a carbamate. The negatively charged carbamate then chelates a magnesium ion (Mg²⁺), which is absolutely essential for catalytic activity. The Mg²⁺ ion plays a vital role in coordinating the substrates, stabilizing transition states during the reaction, and facilitating proton abstraction (RuBisCO, n.d.). This activation process ensures that the enzyme is only active when sufficient CO₂ and Mg²⁺ are available.
2.2.2. Small Subunit (RbcS)
The RbcS subunit, typically 12-18 kDa, does not possess catalytic activity itself. However, it is indispensable for the proper folding, assembly, stability, and optimal function of Form I RuBisCO. In plants, RbcS is nuclear-encoded, synthesized in the cytosol, and then imported into the chloroplast. Its precise role has been a subject of extensive research, with evidence suggesting that it:
- Facilitates Assembly: RbcS acts as a crucial chaperone-like component, guiding the correct assembly of the large subunits into the L8S8 hexadecameric structure. Without RbcS, functional Form I RuBisCO generally cannot be formed in vivo.
- Enhances Stability: It contributes significantly to the overall structural integrity and thermal stability of the enzyme, protecting the large subunits from denaturation and proteolytic degradation.
- Modulates Catalytic Properties: While not directly involved in catalysis, RbcS can subtly influence the kinetic properties of the large subunit, including the specificity factor (S_C/O, discussed below) and the catalytic turnover rate. Different RbcS isoforms within the same plant species, or from different species, can confer slightly different kinetic characteristics to the RuBisCO holoenzyme, highlighting its regulatory role.
The interaction between RbcL and RbcS is extensive, involving multiple contact points that stabilize the overall hexadecameric complex. The small subunits essentially form caps on the ends of the L8 core, providing structural support and potentially influencing the microenvironment around the active sites.
2.3. Enzyme Activation: The Role of Carbamylation and Mg²⁺
For RuBisCO to become catalytically active, it must undergo a crucial activation step. This process, often referred to as ‘carbamylation,’ involves two key components:
- CO₂ Binding: A specific, non-substrate molecule of CO₂ binds reversibly to the ε-amino group of a lysine residue located in the active site of the large subunit (Lys201 in many plant RuBisCOs). This reaction forms a negatively charged carbamate group.
- Mg²⁺ Chelation: The newly formed carbamate then chelates a magnesium ion (Mg²⁺). This Mg²⁺ ion is essential for catalysis. It acts as a co-factor, helping to orient and stabilize the negatively charged enediol intermediate of RuBP and facilitating the subsequent attack by CO₂ or O₂.
This activation step is regulated by factors such as CO₂ and Mg²⁺ concentrations, as well as pH. Under low CO₂ or high O₂ conditions, or in the dark, RuBisCO can become inhibited by the tight binding of RuBP or other sugar phosphates to its active site in the absence of the activating CO₂ and Mg²⁺. This inhibition necessitates the action of a dedicated chaperone protein, RuBisCO activase (Rca), to release these inhibitors and allow for carbamylation and subsequent activation (Frontiers in Molecular Biosciences, 2017).
Many thanks to our sponsor Esdebe who helped us prepare this research report.
3. Function and Mechanism: The Dual-Edged Sword of Catalysis
RuBisCO’s primary function is to catalyze the initial step of carbon fixation in the CBB cycle. However, its catalytic repertoire is characterized by a problematic dual specificity, enabling it to react with both its intended substrate, carbon dioxide (CO₂), and molecular oxygen (O₂). This dual activity defines the two competing reactions: carboxylation and oxygenation.
3.1. The Carboxylation Reaction: The Foundation of Life
The carboxylation reaction is the anabolic cornerstone of photosynthesis, leading to the net assimilation of atmospheric carbon into organic matter. This process begins with RuBisCO facilitating the addition of a CO₂ molecule to the five-carbon sugar phosphate, ribulose-1,5-bisphosphate (RuBP).
Mechanism of Carboxylation:
- Enolization of RuBP: The activated RuBisCO, with its carbamylated lysine and bound Mg²⁺, facilitates the abstraction of a proton from the C3 carbon of RuBP. This forms a reactive 2,3-enediol intermediate of RuBP, which is stabilized by the Mg²⁺ ion within the active site.
- CO₂ Addition: The enediol intermediate then acts as a nucleophile, attacking the electrophilic carbon atom of the CO₂ molecule. This results in the formation of an unstable six-carbon intermediate, 2-carboxy-3-keto-D-arabinitol-1,5-bisphosphate (CA-RuBP).
- Hydration and Cleavage: The CA-RuBP intermediate is rapidly hydrated by a water molecule, followed by an enzymatic cleavage. This cleavage reaction, precisely orchestrated by RuBisCO, yields two molecules of the three-carbon compound 3-phosphoglycerate (3-PGA).
The 3-PGA molecules are then subsequently phosphorylated and reduced within the CBB cycle to form glyceraldehyde-3-phosphate (G3P), which can be used to regenerate RuBP (thus closing the cycle) or be channeled into the synthesis of glucose, sucrose, starch, and other essential organic compounds. This pathway efficiently converts inorganic carbon into the organic building blocks necessary for growth and metabolism in photosynthetic organisms (RuBisCO, n.d.).
3.2. The Oxygenation Reaction: The Photorespiratory Burden
Unfortunately, RuBisCO’s active site cannot perfectly distinguish between CO₂ and O₂. In a competing reaction, molecular oxygen can also bind to the activated enediol intermediate of RuBP, leading to the oxygenation reaction.
Mechanism of Oxygenation:
- Enolization of RuBP: Similar to carboxylation, the enediol intermediate of RuBP is formed.
- O₂ Addition: Instead of CO₂, O₂ binds to and reacts with the enediol intermediate. This oxygenation results in the formation of an unstable hydroperoxide intermediate.
- Cleavage: This intermediate rapidly cleaves to produce one molecule of 3-PGA and one molecule of a two-carbon compound, 2-phosphoglycolate (2-PG) (Photorespiration, n.d.).
Unlike 3-PGA, which is a productive intermediate of the CBB cycle, 2-PG is a potent inhibitor of several key enzymes in the CBB cycle and is highly toxic to the cell. Its accumulation would quickly bring photosynthesis to a halt and cause cellular damage. Therefore, plants have evolved a complex and energetically costly metabolic pathway known as photorespiration (or the photorespiratory carbon oxidation cycle) to detoxify 2-PG and recover some of the carbon.
3.3. The Photorespiratory Pathway: An Energy-Consuming Rescue Mission
Photorespiration is a multi-compartmental pathway involving enzymes located in three different organelles: the chloroplast, the peroxisome, and the mitochondrion. It represents a significant drain on photosynthetic efficiency for several reasons:
- Carbon Loss: For every two molecules of 2-PG processed, one molecule of CO₂ is released in the mitochondrion, representing a direct loss of previously fixed carbon.
- Energy Cost: The pathway consumes ATP and NADPH, which are precious energy currencies generated by the light-dependent reactions of photosynthesis. These resources would otherwise be available for carbon fixation through the CBB cycle.
- Nitrogen Loss (Indirect): The recycling of nitrogen-containing intermediates also has an indirect energetic and resource cost.
Key Steps and Enzymes in Photorespiration:
- Chloroplast:
- 2-phosphoglycolate is dephosphorylated by phosphoglycolate phosphatase to yield glycolate.
- Peroxisome:
- Glycolate is transported into the peroxisome and oxidized by glycolate oxidase to glyoxylate, producing hydrogen peroxide (H₂O₂), which is then broken down by catalase.
- Glyoxylate is then transaminated by glutamate:glyoxylate aminotransferase to glycine, using glutamate as the amino donor.
- Mitochondrion:
- Two molecules of glycine are transported into the mitochondrion. Here, the glycine decarboxylase complex converts them into one molecule of serine, releasing one molecule of CO₂ and one molecule of ammonia (NH₃). This is the primary point of carbon loss.
- The ammonia is subsequently refixed by the glutamine synthetase/glutamate synthase (GS/GOGAT) cycle, consuming ATP and NADPH.
- Peroxisome (return):
- Serine is transported back into the peroxisome and converted to hydroxypyruvate by serine:glyoxylate aminotransferase, then reduced to glycerate by hydroxypyruvate reductase.
- Chloroplast (return):
- Glycerate is transported back into the chloroplast and phosphorylated by glycerate kinase to 3-PGA, re-entering the CBB cycle.
Under typical atmospheric conditions (21% O₂, 0.04% CO₂) and moderate temperatures, photorespiration can reduce the net photosynthetic efficiency of C3 plants by 20-50%. This inefficiency is exacerbated at higher temperatures, as the solubility of CO₂ decreases more rapidly than that of O₂ in water, leading to a higher O₂/CO₂ ratio at the active site, and because RuBisCO’s specificity for CO₂ decreases with increasing temperature.
3.4. Specificity Factor (S_C/O) and Catalytic Turnover Rate (k_cat)
Two critical kinetic parameters define RuBisCO’s efficiency:
- Specificity Factor (S_C/O): This dimensionless ratio quantifies RuBisCO’s preference for CO₂ over O₂. It is defined as (V_c / K_c) / (V_o / K_o), where V_c and V_o are the maximal rates of carboxylation and oxygenation, respectively, and K_c and K_o are the Michaelis constants for CO₂ and O₂. A higher S_C/O indicates a greater discrimination against oxygenation. Plant RuBisCOs typically have S_C/O values ranging from 80-100, while some red algal and cyanobacterial RuBisCOs can have values as high as 120-140 (Proceedings of the National Academy of Sciences of the United States of America, 2020). This variation underscores the potential for improvement.
- Catalytic Turnover Rate (k_cat): Also known as the turnover number, k_cat represents the maximum number of substrate molecules (RuBP + CO₂ or O₂) that a single active site can convert into product per unit time when the enzyme is saturated. For Form I RuBisCO from C3 plants, the k_cat for carboxylation is remarkably slow, typically around 3-10 reactions per second. In contrast, many other enzymes exhibit k_cat values in the hundreds or even thousands of reactions per second. This sluggishness means that plants must produce vast quantities of RuBisCO to achieve sufficient rates of carbon fixation, making it a major nitrogen sink in leaves (The Science Notes, n.d.).
There appears to be an evolutionary trade-off between the specificity factor and the catalytic turnover rate: RuBisCOs with higher S_C/O values often exhibit lower k_cat values, and vice versa. This inverse relationship presents a significant challenge for engineering efforts, as simultaneously improving both parameters has proven difficult.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
4. Evolutionary History: Adapting to a Changing Atmosphere
RuBisCO’s evolutionary trajectory is deeply intertwined with the dramatic changes in Earth’s atmospheric composition, particularly the rise of molecular oxygen. Its long history, spanning billions of years, has shaped its current structure and dual functionality.
4.1. Origins in an Anoxic World
It is widely hypothesized that RuBisCO originated in a primordial Earth largely devoid of free oxygen, an anoxic or anoxic-to-micro-oxic environment. In such conditions, the enzyme would have functioned exclusively as a carboxylase, efficiently fixing CO₂ without the confounding presence of O₂. Early forms of RuBisCO, likely resembling modern Form III or Form II enzymes found in anaerobic bacteria and archaea, would have facilitated carbon fixation for ancient autotrophs (Proceedings of the National Academy of Sciences of the United States of America, 2020). These early catalysts were crucial for the development of primary productivity long before the advent of oxygenic photosynthesis.
4.2. The Great Oxidation Event and the Rise of Oxygenase Activity
The emergence of oxygenic photosynthesis by cyanobacteria, beginning approximately 2.5 to 3 billion years ago, marked a revolutionary turning point in Earth’s history: the Great Oxidation Event (GOE). As oxygen levels gradually increased in the atmosphere and oceans, a new selective pressure was imposed on RuBisCO. O₂, being chemically similar in some respects to CO₂ and sharing a similar binding site, began to compete with CO₂ at the enzyme’s active site. This competition led to the inevitable oxygenation reaction and the production of toxic 2-phosphoglycolate.
From an evolutionary standpoint, the development of the photorespiratory pathway can be viewed as a necessary detoxification mechanism rather than a primary metabolic strategy. It allowed organisms to survive and thrive in an oxygen-rich environment by efficiently scavenging the toxic byproduct of RuBisCO’s promiscuity (Photorespiration, n.d.). While energetically wasteful, photorespiration prevented cellular damage and allowed photosynthesis to continue in the presence of oxygen. This adaptation, though seemingly inefficient, was critical for the widespread success of oxygenic photosynthesis and the diversification of aerobic life.
4.3. Diversification of RuBisCO Forms and Ecological Niches
The different forms of RuBisCO (Form I, II, III, IV) reflect distinct evolutionary lineages and adaptations to various ecological niches and metabolic requirements:
- Form I Dominance: Form I RuBisCO, with its characteristic L8S8 structure, is the predominant form in all oxygenic photosynthetic organisms (plants, algae, cyanobacteria). Its evolution coincided with the rise of oxygenic photosynthesis and the need for a more robust and regulated enzyme. The evolution of the small subunit (RbcS) in Form I enzymes is particularly significant; while not directly catalytic, it is believed to have played a crucial role in enhancing the stability, assembly, and potentially the specificity of the large subunit in an oxygen-rich environment. Form I enzymes are generally characterized by a higher specificity factor for CO₂ compared to Form II enzymes, reflecting an ongoing evolutionary pressure to mitigate photorespiration.
- Form II Persistence: Form II enzymes, lacking the small subunit, persist in certain anaerobic or facultative anaerobic proteobacteria and some dinoflagellates. Their simpler structure might reflect a more ancient origin or adaptation to environments where oxygen competition is less severe or where the enzyme is utilized in an anaplerotic role rather than primary carbon fixation.
- Form III and IV in Anaerobes and Non-fixers: Form III enzymes are found primarily in archaea and some anaerobic bacteria, often in contexts of non-photosynthetic carbon fixation. Form IV (RuBisCO-like proteins) have diverged completely from the catalytic function, repurposing the RuBisCO scaffold for other metabolic roles. These forms highlight the deep evolutionary roots of the RuBisCO protein family and its versatile structural scaffold.
4.4. Adaptations for CO₂ Concentration: C4 and CAM Photosynthesis
In higher plants, the inherent inefficiencies of RuBisCO in the face of rising O₂ levels and warmer temperatures led to the independent evolution of sophisticated carbon concentrating mechanisms (CCMs). These strategies effectively increase the CO₂ concentration around RuBisCO’s active site, thereby suppressing photorespiration:
- C4 Photosynthesis: Evolved independently multiple times in diverse plant lineages (e.g., maize, sugarcane). C4 plants employ a spatial separation of initial carbon fixation and the CBB cycle. CO₂ is initially fixed in mesophyll cells by PEP carboxylase (PEPco), which has no oxygenase activity and a high affinity for bicarbonate. The resulting C4 acid (e.g., malate, aspartate) is then transported to specialized bundle sheath cells, where it is decarboxylated, releasing a high concentration of CO₂. This concentrated CO₂ is then refixed by RuBisCO, operating in an environment with significantly reduced O₂ competition (RuBisCO, n.d.).
- Crassulacean Acid Metabolism (CAM): Common in succulents and desert plants (e.g., cacti, pineapples). CAM plants employ a temporal separation of carbon fixation. Stomata open at night, allowing CO₂ uptake and initial fixation by PEPco into C4 acids, which are stored in the vacuole. During the day, stomata close to conserve water, and the stored C4 acids are decarboxylated, releasing CO₂ internally for refixation by RuBisCO within the chloroplasts. This strategy minimizes water loss while also suppressing photorespiration.
These elaborate CCMs demonstrate nature’s evolutionary workarounds to RuBisCO’s limitations, allowing plants to thrive in environments where CO₂ availability is limited or photorespiration is particularly detrimental. Phylogenetic studies have rigorously traced these evolutionary changes, demonstrating the dynamic interplay between RuBisCO’s inherent properties, atmospheric composition, and the adaptive strategies developed by photosynthetic organisms (Proceedings of the National Academy of Sciences of the United States of America, 2020).
Many thanks to our sponsor Esdebe who helped us prepare this research report.
5. Challenges and Strategies in Engineering RuBisCO: The Quest for Super-Efficient Photosynthesis
The profound impact of RuBisCO’s inefficiencies on global crop productivity and biomass accumulation has spurred intensive research efforts aimed at genetically engineering the enzyme for enhanced performance. However, modifying RuBisCO is a formidable challenge due to its intricate structure, complex activation mechanism, and the delicate balance of its kinetic properties.
5.1. The Engineering Imperative
Improving RuBisCO’s efficiency is central to meeting the escalating demands for food, feed, fiber, and biofuels in a world facing unprecedented population growth and the escalating pressures of climate change. A more efficient RuBisCO could translate into:
- Increased Crop Yields: Enhanced carbon fixation directly leads to more biomass and higher yields.
- Improved Resource Use Efficiency: Better photosynthetic efficiency can reduce the need for water (due to reduced stomatal opening for the same CO₂ uptake), nitrogen (less RuBisCO required), and light.
- Enhanced Climate Resilience: Crops with optimized RuBisCO could perform better under future climate scenarios, including elevated temperatures and CO₂ levels.
5.2. Major Engineering Strategies and Associated Challenges
Research efforts to improve RuBisCO’s performance broadly fall into several interconnected strategies:
5.2.1. Enhancing Catalytic Efficiency: The Speed-Specificity Trade-off
The primary goal here is to increase the carboxylation rate (k_cat) and/or the specificity for CO₂ over O₂ (S_C/O), ideally without compromising the other parameter.
- Rational Design and Site-Directed Mutagenesis: This approach involves identifying specific amino acid residues or structural motifs within the RuBisCO large or small subunits that are hypothesized to influence catalysis or specificity. Based on structural models and comparative sequence analysis of RuBisCOs from various organisms, targeted mutations are introduced. For example, residues near the active site, in loop regions that control substrate access, or at the RbcL-RbcS interface are common targets. While some success has been achieved in bacterial or cyanobacterial RuBisCOs (e.g., slight improvements in S_C/O or k_cat), translating these gains to plant RuBisCOs, which are generally more optimized, has proven exceptionally difficult. The complex interplay of multiple residues means that a mutation improving one property often negatively impacts another (Proceedings of the National Academy of Sciences of the United States of America, 2020).
- Directed Evolution: This involves creating large libraries of mutated RuBisCO genes and then applying selection pressures to identify variants with desired properties. While powerful for simpler enzymes, directed evolution of RuBisCO is hampered by the lack of effective in vivo selection systems in higher plants due to its complex assembly and the essentiality of the enzyme for survival. Surrogate systems, such as expression in E. coli or cyanobacteria, have yielded some improved variants, but their transferability to plant chloroplasts often faces challenges related to folding, assembly, and integration into the plant’s metabolic machinery.
- Heterologous Expression of ‘Better’ RuBisCOs: An appealing strategy is to introduce RuBisCOs from other organisms, particularly cyanobacteria, which often possess higher specificity factors or faster turnover rates than plant RuBisCOs. Efforts have focused on expressing cyanobacterial RuBisCO (e.g., from Synechococcus elongatus) in tobacco chloroplasts. While functional cyanobacterial RuBisCO has been successfully assembled in planta, significant challenges remain, including ensuring proper integration with the plant’s Rca, optimizing its expression levels, and overcoming potential compatibility issues with other chloroplast proteins. The full promise of significantly outperforming the native plant enzyme has yet to be realized in field conditions (The FEBS Journal, 2023).
5.2.2. Compartmentalization: Creating a CO₂-Rich Environment
Nature’s solution to RuBisCO’s oxygenase problem in many photosynthetic bacteria and cyanobacteria is the carboxysome, a protein microcompartment that concentrates CO₂ around RuBisCO. Engineering this natural CO₂-concentrating mechanism (CCM) into higher plant chloroplasts is a major frontier.
- Carboxysomes: These polyhedral bodies encapsulate RuBisCO and carbonic anhydrase (CA). Bicarbonate (HCO₃⁻) is actively transported into the carboxysome, where CA rapidly converts it to CO₂. The protein shell of the carboxysome is permeable to bicarbonate but largely impermeable to CO₂ and O₂, thus trapping a high concentration of CO₂ internally and providing an oxygen-poor environment for RuBisCO. The CO₂ then diffuses to the active sites of the encapsulated RuBisCO (FEBS Letters, 2023).
- Engineering Carboxysomes into Plants: The ambitious goal is to transplant the entire carboxysome system, or a simplified version, into plant chloroplasts. This involves expressing multiple bacterial genes encoding the shell proteins, the carboxysomal carbonic anhydrase, and the specific RuBisCO required to be encapsulated. Significant progress has been made in expressing individual components and even partial carboxysome shells in chloroplasts. However, challenges include ensuring proper assembly of a functional, semi-permeable shell, efficient loading of both RuBisCO and carbonic anhydrase into the shell, and maintaining metabolic flux into and out of the compartment without causing energetic burden or toxicity to the plant (FEBS Letters, 2023).
- Pyrenoids: Some algae utilize pyrenoids, proteinaceous structures rich in RuBisCO, as a form of CCM. While structurally different from carboxysomes, they serve a similar function. Research is exploring the possibility of engineering algal pyrenoid components into higher plants.
5.2.3. Optimizing Chaperone Function and Activation
RuBisCO’s activity is tightly regulated by chaperone proteins, primarily RuBisCO activase (Rca). Enhancing Rca’s efficiency and robustness is another critical engineering target.
- Rubisco Activase (Rca): Rca is an ATP-dependent AAA+ chaperone that ‘activates’ RuBisCO. Its primary roles are to remove inhibitory sugar phosphates (like RuBP) that can bind tightly to the active site in the absence of Mg²⁺ and CO₂, and to facilitate the carbamylation and Mg²⁺ binding necessary for RuBisCO’s catalytic activity. Rca also helps maintain RuBisCO in its active state and can assist in its repair during stress (Frontiers in Molecular Biosciences, 2017).
- Temperature Sensitivity of Rca: A major limitation, particularly in crops, is that Rca is often more heat-sensitive than RuBisCO itself. Under heat stress, Rca can denature or become less efficient, leading to a deactivation of RuBisCO and a sharp decline in photosynthesis, even if RuBisCO itself could tolerate higher temperatures. This contributes to the phenomenon of ‘photoinhibition.’
- Engineering Rca: Strategies include identifying and introducing more thermotolerant Rca variants from heat-adapted species, or using rational design and directed evolution to improve the thermal stability and catalytic efficiency of native Rca. Enhancing Rca activity has been shown to improve photosynthetic rates and growth, especially under fluctuating light or temperature conditions.
- Other Chaperones: The folding and assembly of the complex L8S8 RuBisCO also depend on other chaperonins, such as the Cpn60/Cpn10 system (chloroplast GroEL/GroES homologues). Ensuring the efficient folding and assembly of both native and engineered RuBisCO is crucial, and optimizing these chaperone systems can indirectly enhance RuBisCO function.
5.2.4. Enhancing CO₂ Delivery to the Chloroplast
While not directly modifying RuBisCO, improving the supply of CO₂ to the enzyme’s immediate vicinity can significantly boost its effective activity.
- Carbonic Anhydrase (CA): Overexpressing or spatially targeting CA to the chloroplast stroma or near RuBisCO can accelerate the interconversion of CO₂ and bicarbonate (HCO₃⁻), thus speeding up CO₂ diffusion and ensuring a ready supply to RuBisCO, particularly in aquatic environments or within the carboxysome context.
- Aquaporins: Modifying the permeability of chloroplast membranes to CO₂ through the engineering of aquaporins (water channels that can also transport small gases) could facilitate faster CO₂ diffusion from the cytosol into the chloroplast stroma, reducing diffusional limitations.
- Improving Mesophyll Conductance: Genetic approaches to increase the internal CO₂ conductance from stomata through the mesophyll cells to the chloroplasts could provide more substrate for RuBisCO.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
6. Implications for Agricultural Applications and Future Prospects
The successful engineering of RuBisCO holds transformative potential for agriculture, offering a path towards significantly increasing crop yields and enhancing plant resilience in the face of climate change. However, realizing this potential requires a nuanced understanding of complex biological trade-offs and the integration of multiple biotechnological approaches.
6.1. Boosting Crop Yields and Resource Use Efficiency
As discussed, RuBisCO’s inefficiencies significantly limit photosynthetic productivity in C3 crops (e.g., rice, wheat, soybean, potato), which account for the vast majority of global food production. Estimates suggest that addressing RuBisCO’s limitations could theoretically increase C3 crop yields by 20-50%. This potential yield increase is crucial for feeding a global population projected to reach nearly 10 billion by 2050 (Proceedings of the National Academy of Sciences of the United States of America, 2020).
Beyond just yield, improved RuBisCO efficiency could lead to enhanced resource use efficiency:
- Water Use Efficiency (WUE): If plants can fix carbon more efficiently, they may require less stomatal opening to achieve the same photosynthetic rate, thereby reducing water loss through transpiration. This is particularly critical in drought-prone regions.
- Nitrogen Use Efficiency (NUE): RuBisCO represents a substantial nitrogen sink in leaves. A more efficient enzyme would mean less RuBisCO protein is needed to achieve the same or higher carbon fixation rates, freeing up nitrogen for other vital metabolic processes and potentially reducing the need for nitrogen fertilizer inputs, with environmental benefits.
- Light Use Efficiency (LUE): By reducing photorespiratory losses, more of the absorbed light energy can be productively channeled into carbon fixation.
6.2. Enhancing Climate Change Adaptation and Resilience
Climate change projections include rising atmospheric CO₂ levels, increasing global temperatures, and more frequent extreme weather events. Engineering RuBisCO offers strategies to enhance crop adaptation to these new conditions:
- Elevated CO₂ Response: While higher atmospheric CO₂ generally benefits C3 photosynthesis by increasing the CO₂/O₂ ratio at the active site and suppressing photorespiration, engineering RuBisCO for even greater CO₂ specificity or faster turnover at high CO₂ could unlock further yield gains in future CO₂-enriched environments.
- Heat Stress Tolerance: As temperatures rise, photorespiration increases because RuBisCO’s specificity for CO₂ decreases, and the solubility of CO₂ in water decreases more than that of O₂. Moreover, RuBisCO activase (Rca) is often heat-sensitive. Engineering heat-tolerant RuBisCO and/or Rca variants is crucial to maintain photosynthetic efficiency under increasing temperatures, helping crops cope with heat waves.
- Drought Tolerance: By improving WUE, RuBisCO engineering can indirectly contribute to drought tolerance, as plants can maintain higher photosynthetic rates with less water.
6.3. Navigating Trade-offs and Holistic Plant Engineering
Despite the immense promise, the engineering of RuBisCO is fraught with challenges and necessitates careful consideration of potential trade-offs and unintended consequences. The enzyme’s complex regulation and its deep integration into the cellular metabolism mean that isolated modifications might have pleiotropic effects on other vital processes.
- Speed vs. Specificity Trade-off: As noted, there is an inverse correlation between RuBisCO’s catalytic rate (k_cat) and its specificity factor (S_C/O). Engineering a RuBisCO that is both significantly faster AND more specific remains an elusive goal. A slight increase in specificity might come at the expense of overall speed, requiring plants to produce even more of the enzyme to compensate, negating nitrogen savings.
- Metabolic Burden: Introducing foreign enzymes (e.g., from carboxysomes) or entirely new metabolic pathways can impose a significant energetic and resource burden on the plant. This ‘cost of expression’ must be outweighed by the photosynthetic benefits.
- Assembly and Activation: Heterologous expression of RuBisCO from one species into another (e.g., cyanobacterial RuBisCO in a plant) is challenging because the enzyme often requires specific chaperones and assembly factors that may not be present or compatible in the host organism. Even if assembled, ensuring its proper activation and regulation in the foreign environment is critical.
- Holistic System Engineering: The most successful approaches will likely involve a multi-gene, systems-level engineering strategy rather than targeting RuBisCO in isolation. This might include optimizing other CBB cycle enzymes, improving CO₂ diffusion pathways, enhancing light-harvesting efficiency, and engineering the photorespiratory pathway itself to be less wasteful.
6.4. Current Status and Future Outlook
Significant progress has been made in understanding RuBisCO and developing engineering tools. Major international consortia, such as the Realizing Increased Photosynthetic Efficiency (RIPE) project, are actively pursuing these goals, demonstrating that modest but significant improvements in photosynthetic efficiency have been achieved in model systems (e.g., tobacco). These include optimizing Rca, re-routing photorespiration, and expressing improved RuBisCO variants.
The ultimate vision is to translate these laboratory successes into new varieties of staple crops with fundamentally enhanced photosynthetic performance in the field. This will require continued fundamental research into RuBisCO’s biochemistry and evolution, coupled with advanced synthetic biology, genetic engineering, and high-throughput phenotyping techniques. As biotechnology advances, so too will our capacity to precisely engineer these complex biological systems. While challenges remain substantial, the potential benefits for global food security, climate change mitigation, and sustainable agriculture ensure that the quest for a super-efficient RuBisCO will continue to be a top priority in plant science.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
7. Conclusion
RuBisCO, the most abundant enzyme on Earth, is undeniably central to the global carbon cycle, serving as the indispensable gatekeeper for carbon entry into the biosphere. Its ubiquity across nearly all photosynthetic organisms underscores its fundamental role in sustaining life. Yet, its inherent kinetic limitations – a slow catalytic rate and a problematic oxygenase activity leading to the wasteful process of photorespiration – present a significant bottleneck to photosynthetic efficiency, particularly in agricultural systems. Understanding the enzyme’s intricate multimeric structure, its precise dual-reaction mechanisms, and its profound evolutionary journey in response to Earth’s changing atmospheric conditions provides critical insights into these inefficiencies.
The formidable challenge of engineering RuBisCO for enhanced performance has spurred diverse and innovative biotechnological strategies. These include rational design and directed evolution to improve its intrinsic specificity and turnover, the ambitious prospect of transplanting natural CO₂-concentrating mechanisms like carboxysomes into crop plants, and optimizing the function of essential chaperones like RuBisCO activase. While substantial progress has been made, particularly in model systems, the complexity of the enzyme and the need to integrate modifications within the intricate context of whole-plant physiology necessitate continued, rigorous, and interdisciplinary research. The potential implications for global food security, sustainable agriculture, and climate change adaptation are profound, offering a compelling imperative to overcome the complexities associated with finally unleashing the full photosynthetic potential of this most vital of enzymes.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
References
- FEBS Letters. (2023). The challenge of engineering Rubisco for improving photosynthesis. Retrieved from https://febs.onlinelibrary.wiley.com/doi/full/10.1002/1873-3468.14678
- Frontiers in Molecular Biosciences. (2017). Rubisco Activases: AAA+ Chaperones Adapted to Enzyme Repair. Retrieved from https://www.frontiersin.org/articles/10.3389/fmolb.2017.00020/full
- Photorespiration. (n.d.). In Wikipedia. Retrieved July 15, 2025, from https://en.wikipedia.org/wiki/Photorespiration
- Proceedings of the National Academy of Sciences of the United States of America. (2020). A short history of RuBisCO: The rise and fall (?) of Nature’s predominant CO2 fixing enzyme. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC7610757/
- Proceedings of the National Academy of Sciences of the United States of America. (2020). Biophysical analysis of the structural evolution of substrate specificity in RuBisCO. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC7720200/
- RuBisCO. (n.d.). In Wikipedia. Retrieved July 15, 2025, from https://en.wikipedia.org/wiki/RuBisCO
- The Science Notes. (n.d.). RuBisCO: Discovery, Structure, Characteristics, and Functions. Retrieved from https://thesciencenotes.com/rubisco-discovery-structure-characteristics-functions/


RuBisCO, huh? So it’s the Earth’s most abundant enzyme, but kind of a slacker? Sounds like someone I know. Maybe if we offered it employee of the month, it might pick up the pace. Or perhaps a stern talking-to from a motivational lichen?