Abstract
Diabetes mellitus, a pervasive chronic metabolic disorder characterized by persistent hyperglycemia resulting from either insufficient insulin production or ineffective insulin utilization, demands meticulous insulin management to mitigate the onset and progression of debilitating microvascular and macrovascular complications. For decades, subcutaneous insulin injections have served as the fundamental pillar of diabetes therapy. However, the relentless pace of biomedical and engineering innovation has ushered in an era of advanced insulin delivery systems designed to significantly improve patient adherence, enhance glycemic control, reduce the physical and psychological burden of living with diabetes, and ultimately elevate the overall quality of life for individuals with this condition. This comprehensive report embarks on a detailed exploration of both the established and cutting-edge insulin delivery methodologies currently available or under development. It critically examines traditional approaches, including vials with syringes and insulin pens, before delving into sophisticated technologies such as continuous insulin pumps, digitally integrated smart pens, innovative transdermal patches, pulmonary inhaled insulin, and highly advanced closed-loop automated insulin delivery systems. The discussion encompasses their fundamental mechanisms of action, comparative efficacy in achieving optimal glycemic targets, impact on patient adherence and satisfaction, associated safety profiles, and the transformative future trends poised to reshape diabetes management.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
1. Introduction
Diabetes mellitus represents a global health crisis, affecting hundreds of millions worldwide and imposing immense healthcare burdens. The cornerstone of management for both type 1 diabetes (T1D) and many cases of type 2 diabetes (T2D) is the precise administration of exogenous insulin to regulate blood glucose levels within a physiologically acceptable range. Historically, the discovery of insulin in the early 20th century by Banting and Best revolutionized diabetes care, transforming a fatal diagnosis into a manageable chronic condition. Initially, insulin was administered via crude syringes and vials, a method that, while life-saving, presented numerous practical challenges. These challenges included the inherent pain and discomfort of multiple daily injections, the psychological barrier of needle phobia, the potential for dosing inaccuracies, and the significant impact on daily life and spontaneity.
In response to these persistent challenges, and driven by an understanding that patient adherence is paramount to long-term success, the field of diabetes technology has undergone a profound evolution. This evolution has spanned from incremental improvements in injection devices to the development of highly sophisticated, interconnected systems that can emulate, to varying degrees, the physiological function of a healthy pancreas. This report aims to provide an exhaustive review of this technological progression, offering an in-depth analysis of the mechanisms underpinning each delivery system, their demonstrated efficacy in clinical settings, the critical factors influencing patient acceptance and adherence, and the anticipated trajectory of innovation that promises increasingly personalized and automated diabetes care. By examining the current landscape and future prospects, this report seeks to illuminate the profound advancements enhancing the lives of individuals living with diabetes.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
2. Traditional and Early Advanced Insulin Delivery Methods
For nearly a century, subcutaneous insulin injection remained the sole viable method of insulin delivery. While effective, it paved the way for innovations seeking to improve convenience, accuracy, and patient experience.
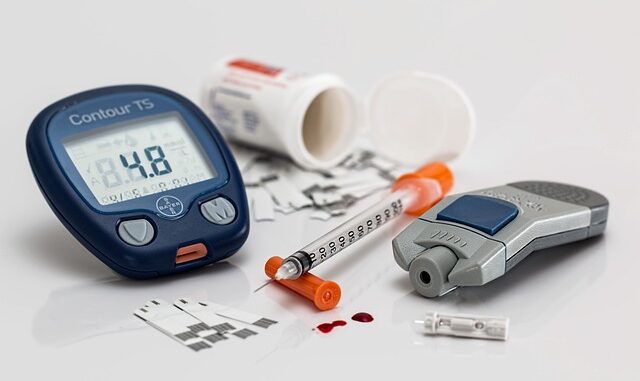
2.1 Insulin Injections: Vials and Syringes
Since the widespread clinical availability of insulin in the 1920s, the administration of insulin via vials and conventional syringes has been the foundational method. This approach involves manually drawing a specific dose of insulin from a glass vial using a sterile syringe and then injecting it subcutaneously, typically into the abdomen, thigh, or upper arm.
Mechanism and Historical Context: Early insulin formulations were primarily animal-derived, and syringe technology was rudimentary. Over time, human insulin, recombinant DNA technology, and insulin analogues (rapid-acting, short-acting, intermediate-acting, and long-acting) were developed, offering improved pharmacokinetic and pharmacodynamic profiles. Syringes also evolved, featuring finer gauges, shorter needles, and clearer markings to facilitate more accurate dosing and reduce discomfort.
Advantages: The primary advantages of vials and syringes include their widespread availability, low cost, and the flexibility they offer in dosing, allowing for precise titration of units. This method is particularly useful in situations where fixed-dose devices are not appropriate or economically viable.
Disadvantages: Despite their longevity, vials and syringes are fraught with numerous disadvantages. These include:
* Dosing Errors: Manual drawing can lead to inaccuracies, particularly for patients with visual impairments or dexterity issues, potentially resulting in hypoglycemia or hyperglycemia.
* Injection Pain and Needle Phobia: The need for multiple daily injections (typically 4-6) is a significant barrier for many, leading to needle phobia and avoidance behaviors that negatively impact adherence.
* Sterility Concerns: Repeated handling of vials and syringes can introduce risks of contamination if not managed properly.
* Portability and Discretion: Carrying vials, syringes, and alcohol swabs can be cumbersome and less discreet, affecting a patient’s social life and willingness to administer insulin in public settings.
* Waste: Each injection typically requires a new syringe, contributing to medical waste.
2.2 Insulin Pens
Insulin pens emerged as a significant improvement over vials and syringes, addressing many of the practical limitations. Introduced in the 1980s, these devices resemble a large fountain pen and have become the dominant method for insulin injection globally. (en.wikipedia.org/wiki/Injector_pen)
Mechanism and Evolution: Insulin pens contain pre-filled cartridges or are disposable units containing a fixed amount of insulin. A dial on the pen allows users to select the desired dose, which is then administered by depressing a button, pushing a plunger, or twisting a knob. Disposable needles are attached to the pen for each injection.
Early pens were simple mechanical devices. Subsequent generations introduced features like memory functions to recall the last dose administered or the time of the last injection. The evolution of pens also saw improvements in ergonomic design, auditory and tactile feedback for dose delivery, and compatibility with various insulin types and needle lengths.
Advantages:
* Improved Dosing Accuracy: Pens deliver precise doses, reducing the likelihood of manual drawing errors.
* Ease of Use: They are simpler to operate, requiring less dexterity than drawing insulin from a vial.
* Portability and Discretion: Their compact size and pen-like appearance make them highly portable and less conspicuous than traditional syringes, fostering greater social acceptance and adherence.
* Reduced Needle Phobia: While still involving a needle, the process is generally perceived as less intimidating than a syringe.
* Built-in Safety Features: Many pens incorporate features to prevent accidental over-dosing or to ensure proper needle attachment.
Disadvantages: Despite their widespread adoption, insulin pens still have limitations. They require manual administration, meaning patients must remember to inject multiple times daily. They do not automatically track insulin on board, nor do they offer real-time data logging for dose history unless they are ‘smart’ pens. Furthermore, for some individuals, issues related to needle phobia or injection-site discomfort persist. The fixed incremental dosing of some pens (e.g., 0.5 or 1 unit increments) may not allow for the precise micro-dosing sometimes required, especially for very sensitive individuals or young children.
2.3 Insulin Pumps
Insulin pumps represent a substantial leap in insulin delivery technology, transitioning from intermittent injections to continuous subcutaneous insulin infusion (CSII). First developed in the late 1970s and gaining wider acceptance in the 1990s, insulin pumps aim to mimic the physiological insulin secretion patterns of a healthy pancreas more closely than multiple daily injections. (en.wikipedia.org/wiki/Insulin_pump)
Mechanism: An insulin pump is a small, battery-operated, computerized device worn externally on the body (often clipped to clothing or worn in a pocket). It holds a reservoir of rapid-acting insulin. A thin plastic tube (infusion set) connects the pump to a cannula (small, flexible tube) inserted under the skin, typically in the abdomen, thigh, or buttocks. The pump delivers insulin in two primary ways:
* Basal Insulin: A continuous, low rate of insulin delivery throughout the day and night to cover metabolic needs between meals and during sleep. This basal rate can be programmed with varying profiles to match individual physiological requirements (e.g., higher rates in the morning, lower overnight).
* Bolus Insulin: On-demand doses administered before meals or to correct high blood glucose levels. The pump’s bolus calculator can assist patients by suggesting doses based on carbohydrate intake, current blood glucose, and programmed insulin-to-carbohydrate ratios and insulin sensitivity factors.
Types of Pumps:
* Tethered Pumps: The traditional design, where the pump is connected to the infusion site by tubing. Examples include Medtronic MiniMed, Tandem t:slim X2.
* Patch Pumps: Tubeless pumps that adhere directly to the skin at the infusion site, with the reservoir and cannula integrated into a single unit, controlled wirelessly by a separate personal diabetes manager (PDM). The Omnipod system is a prominent example. These offer enhanced discretion and freedom of movement.
Advantages:
* Superior Glycemic Control: Pumps can provide much finer control over insulin delivery than injections, leading to lower HbA1c levels, reduced glycemic variability, and decreased incidence of hypoglycemia.
* Flexibility in Lifestyle: Users can eat meals at irregular times and manage exercise more easily by adjusting basal rates or delivering precise boluses.
* Elimination of Multiple Daily Injections: Patients only need to change their infusion set every 2-3 days, significantly reducing the number of needle sticks.
* Precision Dosing: Pumps can deliver insulin in very small increments (e.g., 0.025 or 0.05 units), allowing for highly individualized dosing, especially beneficial for children and sensitive adults.
Disadvantages:
* Cost: Insulin pumps and their consumables (infusion sets, reservoirs) are significantly more expensive than multiple daily injections, posing a barrier to access.
* Risk of Diabetic Ketoacidosis (DKA): A pump malfunction (e.g., occlusion, insulin degradation) can rapidly lead to DKA due to the lack of long-acting insulin in the system.
* Infusion Site Issues: Skin irritation, infections, or cannula dislodgement can occur at the infusion site.
* Body Attachment: Wearing a device 24/7 can be bothersome, affecting clothing choices, intimacy, and sleep. Patch pumps mitigate this to some extent.
* Learning Curve and Technical Issues: Effective pump use requires significant training and ongoing management, and users must be proficient in troubleshooting.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
3. Alternative and Emerging Insulin Delivery Systems
The quest for less invasive, more convenient, and more intelligent insulin delivery has spurred innovation beyond traditional injections and continuous subcutaneous pumps.
3.1 Inhaled Insulin
Inhaled insulin offers a needle-free alternative for mealtime insulin delivery, leveraging the vast surface area of the lungs for rapid absorption. Its history is marked by both pioneering efforts and significant commercial challenges.
Exubera: A Pioneering Effort: The first inhaled insulin, Exubera (manufactured by Pfizer), received FDA approval in 2006. It promised a revolutionary needle-free experience for prandial insulin. However, despite its initial promise, Exubera was withdrawn from the market in 2007 due to poor sales and concerns regarding its impact on pulmonary function, including a potential association with lung cancer in a small number of patients (though causality was never definitively established), and the large, cumbersome size of the inhaler device. The perception of pulmonary risk and the practical inconveniences overshadowed its benefit.
Afrezza: The Second Generation: A more recent inhaled insulin, Afrezza (manufactured by MannKind Corporation), gained FDA approval in 2014. Afrezza utilizes a compact, credit card-sized inhaler and a formulation of insulin (Technosphere insulin) that forms a dry powder designed for deep lung delivery and rapid absorption. Its pharmacokinetic profile mimics the natural physiological insulin spike after a meal more closely than subcutaneous rapid-acting insulin, leading to a quicker onset of action and shorter duration.
Mechanism of Action: When inhaled, Afrezza powder disperses into fine particles that are absorbed rapidly across the alveolar epithelium into the bloodstream. This rapid absorption allows for insulin to begin acting within minutes (peaking around 12-15 minutes), aligning well with the post-prandial glucose surge.
Clinical Efficacy and Patient Experience: Clinical studies have demonstrated that Afrezza is effective for controlling post-meal blood glucose excursions in both type 1 and type 2 diabetes. For children with type 1 diabetes, a Reuters report indicated that Afrezza was found to be as effective as injected insulin for mealtime coverage, with additional benefits of less weight gain compared to injected insulin and higher satisfaction reported by both children and their parents, likely due to the needle-free aspect and rapid action. (reuters.com/business/healthcare-pharmaceuticals/health-rounds-inhaled-insulin-good-injection-children-mealtime-2025-06-25/) This rapid action also contributes to a reduced risk of delayed post-meal hypoglycemia, as its effect dissipates more quickly.
Side Effects and Considerations: The most common side effect of Afrezza is a cough, which is typically mild and transient. Due to the pulmonary delivery, patients require baseline and periodic monitoring of lung function (Spirometry). Afrezza is contraindicated in patients with chronic lung diseases such as asthma or chronic obstructive pulmonary disease (COPD) and in those who smoke. Its role is primarily for mealtime insulin, meaning patients still require a basal insulin (injected or pumped) to manage their background glucose levels.
Challenges and Future: Despite its advantages, Afrezza’s market penetration has been limited. Challenges include physician and patient familiarity, the need for pulmonary monitoring, and the perception of inhaled therapies. However, its rapid action and needle-free nature keep the concept of inhaled insulin a valuable area of research for certain patient populations.
3.2 Smart Insulin Pens
Smart insulin pens represent a significant digital enhancement to the traditional insulin pen, bridging the gap between manual injection devices and fully automated systems. These reusable devices integrate Bluetooth technology to connect seamlessly with mobile applications, transforming insulin administration from a manual act into a data-rich process.
Technology and Features: Smart pens are equipped with sensors that automatically record key injection data, including:
* Dose Amount: The exact number of units administered.
* Time and Date: The precise timestamp of each injection.
* Insulin Type: Differentiating between rapid-acting and basal insulin injections if using two pens.
This data is then wirelessly transmitted to a compatible smartphone or tablet application. The accompanying mobile apps serve as comprehensive diabetes management platforms, offering advanced features:
* Dose Tracking and History: A clear, chronological record of all insulin doses, eliminating the need for manual logging and reducing recall bias.
* Reminders: Customizable alerts for missed doses, upcoming injections, or glucose checks.
* Insulin on Board (IOB) Calculation: Estimates the amount of active insulin remaining in the body from previous boluses, helping to prevent insulin stacking and subsequent hypoglycemia.
* Integration with Continuous Glucose Monitors (CGM): Many smart pen apps can import CGM data, allowing users to see glucose trends alongside their insulin doses, providing a more holistic view of their diabetes management. (en.wikipedia.org/wiki/Dexcom_CGM)
* Dose Calculator and Bolus Advisor: Some advanced apps incorporate algorithms that suggest insulin doses based on carbohydrate intake, current glucose levels, and individualized insulin sensitivity factors and insulin-to-carb ratios, similar to pump bolus calculators.
* Data Sharing: Facilitates easy sharing of data with healthcare providers, enabling more informed clinical decisions and supporting telemedicine consultations.
Benefits for Patients and Clinicians:
* Enhanced Adherence: By automating dose logging and providing reminders, smart pens significantly improve patient adherence to their prescribed insulin regimen. This reduces forgotten doses and helps identify patterns of non-adherence.
* Reduced Dosing Errors: Real-time feedback and dose calculation assistance minimize the risk of under- or over-dosing.
* Improved Glycemic Control: The availability of comprehensive data allows patients and clinicians to identify trends, understand the impact of insulin on glucose levels, and make timely adjustments to therapy, leading to better Time in Range (TIR) and lower HbA1c.
* Empowerment and Education: Patients gain a deeper understanding of their diabetes patterns and the effects of their actions, fostering greater self-management.
* Streamlined Clinical Review: Clinicians receive organized, accurate data, enabling more efficient and effective consultations.
Limitations and Considerations: While highly beneficial, smart pens are not without limitations. They still require manual injection, meaning they don’t eliminate the needle stick. Their effectiveness relies on consistent user engagement with the app and a smartphone. Cost can be a barrier for some, and data privacy is a crucial concern requiring robust security measures. Furthermore, they are primarily focused on insulin delivery and data logging, rather than active glucose sensing or automated insulin adjustment, which distinguishes them from closed-loop systems.
3.3 Insulin Patches
Insulin patches represent a diverse category of wearable devices designed to deliver insulin transdermally or subcutaneously through a simplified, often more discreet, mechanism than traditional pumps or injections. The concept aims to combine the continuous delivery of a pump with the simplicity of a patch.
3.3.1 Basal Insulin Patches (e.g., V-Go)
These patches are a simpler form of wearable insulin delivery device, primarily designed for patients requiring a continuous basal rate of insulin with bolus options for meals. They are distinct from the more complex ‘smart’ or glucose-responsive patches.
Mechanism: Devices like the V-Go (Valeritas, now Zealand Pharma) are disposable, wearable insulin delivery devices applied to the skin (e.g., abdomen). They contain a pre-filled reservoir of insulin and are designed to deliver a continuous, pre-set basal rate of insulin over 24 hours. Users can also administer bolus doses for meals by pressing a button on the device. They are replaced daily.
Advantages:
* Simplicity: Much simpler to operate than traditional insulin pumps, with fewer settings and programming requirements.
* Needle-Free (Daily): While requiring an initial insertion, the needle is immediately retracted upon application, and the device remains on for 24 hours, reducing the frequency of needle sticks compared to multiple daily injections.
* Discretion: The small, low-profile design is less noticeable than a tethered pump.
* Fixed Basal Rate: Can be beneficial for patients with relatively stable insulin needs, reducing the complexity of pump programming.
Limitations:
* Fixed Basal Rates: Lack the flexibility to adjust basal rates throughout the day or night, which can be a disadvantage for patients with variable insulin needs (e.g., dawn phenomenon).
* Limited Bolus Flexibility: Bolus doses are often in fixed increments, offering less precision than advanced pumps.
* Daily Replacement: Requires daily application and disposal, which can be costly and generate waste.
3.3.2 Smart Insulin Patches (Glucose-Responsive Systems)
This is a highly innovative and largely investigational area of research, aiming to create truly ‘smart’ patches that can sense glucose levels and release insulin automatically, mimicking the body’s natural insulin secretion without external control or input. (en.wikipedia.org/wiki/Smart_insulin_patch)
Mechanism and Underlying Technologies: The core concept revolves around integrating a glucose sensing component with an insulin delivery mechanism within a single, autonomous patch. Various technologies are being explored:
* Microneedle Patches: These patches contain arrays of tiny, hair-thin needles (microneedles) that penetrate only the outermost layer of the skin (stratum corneum) without reaching nerve endings, making the delivery process pain-free. These microneedles can be designed to:
* Contain Glucose-Sensitive Materials: Microneedles are loaded with insulin encapsulated in glucose-responsive polymers or hydrogels. When blood glucose levels rise, the glucose reacts with enzymes (e.g., glucose oxidase) embedded within the polymer matrix, triggering a chemical or physical change (e.g., pH change, swelling/shrinking) that causes the polymer to degrade or release insulin.
* Deliver Pre-Programmed Doses: Simpler microneedle patches could deliver basal insulin or user-triggered boluses without glucose responsiveness, acting as a less invasive form of subcutaneous delivery.
* Transdermal Delivery Enhancements: While traditional transdermal delivery of large molecules like insulin is challenging due to the skin’s barrier function, researchers are exploring:
* Iontophoresis: Using a small electrical current to drive insulin molecules across the skin.
* Sonophoresis: Using ultrasound waves to temporarily increase skin permeability.
* Chemical Enhancers: Topical agents that reversibly disrupt the skin barrier. (jpionline.org/10.4103/2230-973x.176456) However, achieving consistent and sufficient insulin delivery rates for physiological needs via these non-microneedle transdermal methods remains a significant hurdle due to insulin’s large molecular size and the need for tight glucose control.
Potential Benefits:
* True Automation: The ultimate goal is a fully automated ‘closed-loop’ system on a patch, eliminating manual injections and continuous monitoring.
* Pain-Free Delivery: Microneedles are designed to be painless.
* Mimicking Natural Physiology: The glucose-responsive release aims to closely mimic the body’s natural insulin secretion, potentially reducing both hyperglycemia and hypoglycemia.
* Reduced Burden: Simplifies diabetes management significantly, improving quality of life.
* Discretion: A small, wearable patch would be highly discreet.
Challenges and Future Outlook: Despite the immense potential, smart insulin patches face considerable technical and regulatory challenges:
* Reliability and Accuracy: Ensuring consistent and accurate glucose sensing and insulin release in a dynamic physiological environment.
* Insulin Stability: Maintaining insulin stability and potency within the patch over its wear time.
* Biocompatibility and Immune Response: Ensuring the materials used are biocompatible and do not elicit adverse immune reactions.
* Scalability and Manufacturing: Developing cost-effective methods for mass production.
* Lag Time: The time taken for the patch to sense glucose changes and release insulin effectively needs to be minimized for optimal post-meal control.
Research into smart insulin patches is primarily in preclinical and early clinical stages, with successful prototypes demonstrated in animal models. The transition to human trials and widespread clinical availability requires overcoming these significant scientific and engineering hurdles.
3.4 Closed-Loop Systems (Artificial Pancreas)
Closed-loop insulin delivery systems, commonly referred to as ‘artificial pancreas’ systems, represent the pinnacle of automated diabetes management to date. These sophisticated systems integrate three core technologies to autonomously adjust insulin delivery in real-time based on continuous glucose monitoring data, thereby aiming to maintain glucose levels within a target range and significantly reduce the burden of manual adjustments. (iomcworld.com/open-access/transforming-diabetes-management-the-latest-in-insulin-delivery-systems-130856.html)
Core Components and Mechanism: A closed-loop system comprises:
* Continuous Glucose Monitor (CGM): A small sensor inserted under the skin measures interstitial glucose levels every few minutes, transmitting data wirelessly. This provides continuous, real-time glucose readings and trend information.
* Insulin Pump: A standard insulin pump, capable of delivering precise micro-boluses and basal insulin, acts as the effector, receiving commands from the control algorithm.
* Control Algorithm: This is the ‘brain’ of the system, typically residing on a smartphone, a dedicated controller, or directly within the pump. The algorithm continuously receives glucose data from the CGM, processes it, predicts future glucose trends, and then instructs the insulin pump to adjust insulin delivery (increase, decrease, or suspend basal insulin, or deliver micro-boluses) to keep glucose within the desired range. These algorithms often employ advanced control strategies such as Proportional-Integral-Derivative (PID) control, Model Predictive Control (MPC), or fuzzy logic.
Evolution of Closed-Loop Systems:
* Early Research Systems: Initially, closed-loop systems were developed in research settings, often requiring multiple devices and external computing power.
* Hybrid Closed-Loop Systems: The first generation of commercially available systems are ‘hybrid’ closed-loop. This means they automate basal insulin delivery and make automatic corrections for high glucose, but still require the user to manually announce meals (carb counting) and confirm boluses. Examples include Medtronic MiniMed 670G/780G, Tandem Control-IQ, and Omnipod 5. These systems have demonstrated significant improvements in Time in Range (TIR) and reduction in hypoglycemia. (en.wikipedia.org/wiki/Automated_insulin_delivery_system)
* Advanced Hybrid and Future Systems: Ongoing development aims for less reliance on manual meal announcements (advanced algorithms learning individual meal patterns), full automation, and potentially multi-hormone systems (e.g., incorporating glucagon to more effectively manage hypoglycemia).
Benefits:
* Improved Glycemic Control: Closed-loop systems consistently demonstrate superior glycemic control compared to manual insulin regimens, leading to lower HbA1c levels, increased Time in Range, and significant reductions in hypoglycemia (especially nocturnal hypoglycemia) and hyperglycemia.
* Reduced Burden of Management: By automating many insulin adjustments, these systems alleviate a substantial portion of the daily mental burden associated with diabetes management, improving sleep quality and reducing diabetes-related distress.
* Enhanced Safety: Proactive adjustments and automatic suspension of insulin delivery during predicted hypoglycemia enhance patient safety.
* Flexibility: Allows for more flexibility in lifestyle, exercise, and meal timing compared to traditional methods.
Challenges:
* Cost and Accessibility: The high cost of CGMs, pumps, and their consumables remains a significant barrier to widespread adoption, particularly in lower-income settings.
* Technological Reliance: Patients become highly dependent on the proper functioning of multiple interconnected devices. System failures (e.g., sensor inaccuracies, pump occlusions) can be critical.
* User Training and Education: Effective use requires comprehensive training and ongoing education to manage the system, troubleshoot, and understand its nuances.
* Algorithm Limitations: Current algorithms still face challenges in perfectly predicting and responding to highly variable physiological events like intense exercise or unpredictable meals.
* Cybersecurity: As these systems become more connected, cybersecurity concerns related to data privacy and device integrity emerge.
* Lag Time: There is an inherent physiological lag between glucose changes in blood and interstitial fluid (where CGM measures), and another lag in subcutaneous insulin absorption, which algorithms must try to account for.
Closed-loop systems represent a transformative step towards truly hands-off diabetes management. While not yet a ‘cure’, they are significantly improving the lives of individuals with diabetes by automating complex decisions and optimizing glucose control around the clock.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
4. Comparative Analysis: Efficacy, Safety, and Patient Adherence
The choice of insulin delivery method is highly individualized, depending on a patient’s diabetes type, lifestyle, technical aptitude, financial resources, and personal preferences. A comparative analysis of efficacy, safety, and patient adherence helps to inform these critical decisions.
4.1 Efficacy
Efficacy is primarily measured by the ability to achieve optimal glycemic control, characterized by low HbA1c levels, increased Time in Range (TIR), and reduced glycemic variability (fluctuations between high and low glucose).
- Vials and Syringes/Basic Pens: Offer the least precise control. While effective when diligently managed, they are prone to human error and lack the continuous adjustment capabilities of more advanced systems. HbA1c and TIR can vary widely based on patient adherence and education.
- Insulin Pumps (CSII): Generally offer superior glycemic control compared to multiple daily injections (MDI). Studies consistently show that CSII leads to lower HbA1c levels and reduced rates of severe hypoglycemia, especially nocturnal hypoglycemia, due to the ability to precisely tailor basal rates and deliver accurate boluses. The flexibility in dosing and timing allows for better post-meal control and reduced glycemic excursions.
- Inhaled Insulin (Afrezza): Primarily impacts post-prandial glucose excursions due to its rapid onset and short duration of action. For mealtime insulin, it can be as effective as injected rapid-acting insulin. Its primary efficacy benefit is often perceived in terms of convenience and speed of action, potentially leading to better adherence to mealtime boluses. However, it does not replace basal insulin, and its overall impact on HbA1c depends on the efficacy of the concomitant basal regimen.
- Smart Insulin Pens: Do not inherently change the pharmacology of insulin delivery but significantly improve the management of injections. By improving adherence, reducing dosing errors, and providing actionable data insights, smart pens can indirectly lead to improved glycemic control (lower HbA1c, increased TIR) compared to traditional pens. They empower patients and clinicians to identify patterns and adjust therapy more effectively.
- Closed-Loop Systems (Artificial Pancreas): Currently represent the gold standard for glycemic control. Hybrid closed-loop systems have demonstrated significant improvements across all key glycemic metrics. For instance, the Medtronic MiniMed 670G, one of the first hybrid closed-loop systems, showed improved glycemic control and reduced hypoglycemic events, particularly overnight. Subsequent systems like Tandem Control-IQ and Omnipod 5 have built on this, achieving even higher Times in Range (e.g., often exceeding 70-80%) and dramatically reducing time spent in hypoglycemia. These systems reduce decision fatigue for users, leading to more consistent and tighter glucose management, especially during challenging periods like sleep or exercise. The algorithms’ predictive capabilities proactively mitigate hyper and hypoglycemia, offering a level of control unparalleled by other methods.
4.2 Safety
Each delivery method carries its own safety considerations:
- Injections (Vials & Pens): Risks include injection site reactions (lipohypertrophy, bruising), accidental needle sticks, and dosing errors leading to hypo/hyperglycemia.
- Insulin Pumps: Primary safety concerns revolve around the risk of Diabetic Ketoacidosis (DKA) if the pump malfunctions (e.g., infusion set occlusion) or if insulin delivery is interrupted for an extended period, as there is no long-acting insulin ‘reservoir’ in the body. Infusion site infections and skin irritation are also potential issues.
- Inhaled Insulin: The main safety concern is the potential impact on pulmonary function. While Afrezza has a better safety profile than Exubera, ongoing spirometry is required, and it’s contraindicated in individuals with pre-existing lung conditions. Cough is a common side effect.
- Smart Insulin Pens: Generally safe, as they mostly enhance the management of existing injection methods. Risks are similar to traditional pens but potentially reduced by better adherence and reduced errors. Data privacy and security become relevant concerns.
- Closed-Loop Systems: While designed to enhance safety by reducing hypoglycemia, risks include algorithm errors (e.g., false CGM readings leading to incorrect insulin delivery), pump malfunctions (similar to traditional pumps, leading to DKA risk), and cybersecurity vulnerabilities. User training is crucial to prevent misuse or misunderstanding of system limitations.
4.3 Patient Adherence and Quality of Life
Patient adherence is arguably the most critical factor for successful diabetes management, and it is profoundly influenced by the convenience, comfort, and perceived burden of the chosen delivery method. Quality of life (QoL) encompasses physical, psychological, and social well-being.
- Vials and Syringes: Lowest adherence due to pain, needle phobia, inconvenience, and social stigma. Significant negative impact on QoL due to daily burden and limitations.
- Insulin Pens: Marked improvement over syringes. Their portability, discretion, and ease of use significantly boost adherence and QoL. Many patients prefer pens due to their simplicity and minimal learning curve. However, they still require conscious effort for each injection.
- Insulin Pumps: While offering superior glycemic control, the need to wear a device 24/7, the cost, and the learning curve can affect adherence and QoL for some individuals. For others, the freedom from multiple injections and improved control vastly improves their QoL. Patch pumps often enhance adherence due to their greater discretion and freedom from tubing.
- Inhaled Insulin: Its needle-free nature is a significant draw, potentially improving adherence to mealtime boluses for those with needle phphobia. However, the need for a separate basal insulin, potential cough, and pulmonary monitoring might impact long-term adherence for some.
- Smart Insulin Pens: By reducing mental burden (no manual logging, reminders) and providing actionable insights, smart pens significantly enhance adherence and empower patients, leading to improved QoL for those comfortable with technology. They bridge the gap for patients not ready or eligible for pumps.
- Insulin Patches (Basal): Simplicity and reduced injection frequency can improve adherence, especially for patients overwhelmed by complex regimens.
- Closed-Loop Systems: While demanding initial training, the reduction in daily diabetes burden, improved sleep quality (less fear of nocturnal hypoglycemia), and superior glucose control contribute to a substantial improvement in quality of life for many users. The psychological relief from constant glucose monitoring and insulin adjustment is a major benefit. However, the continuous attachment of devices and the financial strain can still be barriers for some.
In summary, there is a clear trend towards improved efficacy, safety, and adherence with more technologically advanced systems. However, the ‘best’ method is ultimately the one that the individual patient can consistently use effectively and that best fits their lifestyle and preferences, balancing the benefits of advanced technology with its inherent complexities and costs.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
5. Future Trends in Insulin Delivery
The landscape of insulin delivery is dynamic, driven by unmet needs and breakthroughs in biosensing, materials science, artificial intelligence, and personalized medicine. The future promises even more seamless, intelligent, and less burdensome solutions for diabetes management.
5.1 Personalized Medicine
The concept of ‘one-size-fits-all’ in diabetes management is rapidly being replaced by highly individualized approaches. Future insulin delivery systems will leverage comprehensive data from various sources to tailor therapy to each patient’s unique physiological profile, lifestyle, and genetic predispositions.
Integration of Multi-Omics Data: Beyond glucose levels, future systems may incorporate genomic, proteomic, and metabolomic data to predict individual responses to insulin, identify specific risk factors for complications, and optimize dosing strategies.
AI-Driven Phenotyping: Artificial intelligence and machine learning will be used to analyze vast datasets (glucose, diet, exercise, stress, sleep patterns) to identify distinct physiological phenotypes of diabetes, allowing for highly customized insulin delivery algorithms.
Adaptive Algorithms: Closed-loop systems will become even more adaptive, learning from an individual’s past responses to meals, exercise, and stress, continuously refining their predictive models for unparalleled glycemic precision. They will be able to ‘personalize’ their control logic over time as they learn more about the user.
Lifestyle Integration: Devices will seamlessly integrate with other health trackers (wearables for activity, sleep, heart rate) and potentially even smart home devices to provide a more holistic understanding of a patient’s daily context, allowing for proactive insulin adjustments.
5.2 Non-Invasive Delivery Methods
The holy grail of insulin delivery remains a truly non-invasive method that eliminates the need for any needles or skin penetration. While significant scientific challenges remain, research continues across several fronts:
Oral Insulin: Delivering insulin orally is highly challenging because insulin is a protein and would be degraded by stomach acids and digestive enzymes before it can be absorbed effectively. Furthermore, its large molecular size makes absorption across the intestinal lining difficult. Current research focuses on:
* Encapsulation Technologies: Developing protective coatings (e.g., nanoparticles, microemulsions, liposomes) to shield insulin from degradation in the gastrointestinal tract.
* Permeation Enhancers: Incorporating excipients that temporarily increase the permeability of the intestinal wall to facilitate absorption.
* Colon-Specific Delivery: Designing formulations that release insulin only in the colon, where enzymatic activity is lower. While promising preclinical data exists, clinical translation faces hurdles related to bioavailability consistency and dose predictability. (biomedres.us/fulltexts/BJSTR.MS.ID.004161.php)
Buccal/Sublingual Insulin: Delivery through the oral mucosa (cheeks or under the tongue) offers a potential pathway, bypassing first-pass metabolism in the liver. However, the limited surface area and barrier properties of the mucosa present challenges for sufficient and rapid absorption.
Nasal Insulin: While some nasal insulin formulations have been tested, absorption variability, potential for nasal irritation, and insufficient bioavailability for physiological needs have limited their widespread use.
Ocular and Rectal Insulin: These routes are generally considered less patient-friendly and face similar bioavailability and consistency challenges as other non-invasive methods, though research continues at a very preliminary level.
Once-Weekly Insulin: A promising non-invasive approach (though still subcutaneous for now) aims to reduce injection frequency. Eli Lilly has shown once-weekly insulin appears as effective as daily injections, potentially revolutionizing adherence for basal insulin users. (reuters.com/business/healthcare-pharmaceuticals/health-rounds-once-weekly-insulin-eli-lilly-appears-effective-daily-injections-2024-09-11/)
5.3 Artificial Intelligence and Machine Learning
AI and ML are not merely features but foundational elements of next-generation insulin delivery, moving beyond rule-based programming to predictive and adaptive control.
Enhanced Predictive Capabilities: ML algorithms can analyze vast amounts of glucose data, meal entries, exercise logs, and even stress indicators to predict future glucose trends with greater accuracy than current algorithms. This allows for more proactive and precise insulin adjustments, minimizing glycemic excursions.
Optimization of Closed-Loop Algorithms: AI can continuously optimize the parameters of closed-loop algorithms, making them more robust and personalized. For instance, reinforcement learning could allow a system to ‘learn’ the optimal insulin delivery strategy for an individual over time based on their unique responses.
Pattern Recognition for Proactive Care: AI can identify subtle patterns in glucose data that might indicate issues with insulin sensitivity, absorption, or lifestyle factors, alerting patients or healthcare providers to potential problems before they escalate.
Automated Decision Support: AI will further enhance dose calculators, offering more sophisticated recommendations that account for complex variables beyond simple carbohydrate counting, such as meal composition (fat, protein), previous exercise, and stress levels.
Integration with Big Data: ML can analyze population-level data to identify best practices, optimize treatment guidelines, and even assist in drug discovery and development for novel insulin formulations. (frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1566743/full)
5.4 Other Emerging Technologies and Considerations
- Multi-Hormone Systems: Beyond insulin, researchers are exploring systems that also deliver glucagon to prevent or treat hypoglycemia, or even amylin analogues to improve satiety and post-meal glucose control, mimicking the pancreas’s natural multi-hormone release.
- Bio-Integrated Devices: Long-term implantable sensors and insulin delivery devices that minimize external components and reduce the burden of daily management. Research includes implantable pumps and glucose sensors that can remain in place for months or even years.
- Cell Encapsulation and Islet Transplantation: While not strictly ‘delivery’ in the sense of external devices, significant progress in encapsulating insulin-producing islet cells to protect them from the immune system offers the potential for a biological ‘cure’ that would render external insulin delivery unnecessary.
- Cost and Accessibility: As technologies advance, ensuring equitable access globally remains a critical challenge. Future efforts must focus on developing affordable solutions and advocating for comprehensive insurance coverage.
- Regulatory Landscape: Novel and complex technologies require robust regulatory frameworks to ensure safety and efficacy, which can sometimes slow innovation from reaching patients.
- Psychosocial Support: As technology becomes more central, the need for integrated psychosocial support to address device burden, tech-fatigue, and mental health challenges associated with chronic disease management will grow.
The trajectory of insulin delivery technology is one of increasing automation, intelligence, and personalization. While complete automation and non-invasive methods are still on the horizon, the continuous advancements promise a future where diabetes management is significantly less burdensome and more effective for millions worldwide. (journals.lww.com/ijdt/fulltext/2023/02010/advancements_in_insulin_delivery_technology__a.5.aspx)
Many thanks to our sponsor Esdebe who helped us prepare this research report.
6. Conclusion
The evolution of insulin delivery systems reflects a remarkable journey from rudimentary injections to highly sophisticated, intelligent, and interconnected technologies. What began as a life-saving but burdensome necessity has transformed into an increasingly nuanced and patient-centric aspect of diabetes care. Traditional methods, such as vials with syringes and basic insulin pens, remain prevalent due to their accessibility and simplicity, serving as essential tools for millions. However, their limitations in terms of precision, convenience, and data insights have paved the way for groundbreaking innovations.
Advanced systems like continuous insulin pumps have revolutionized glycemic control by offering precise, continuous, and highly adaptable insulin delivery, significantly reducing the frequency of injections and enhancing lifestyle flexibility. The advent of smart insulin pens has further digitized and simplified the injection experience, providing invaluable data logging and decision support that directly impacts patient adherence and optimizes therapeutic outcomes. While nascent, the promise of inhaled insulin offers a needle-free prandial option, addressing a key psychological barrier for many, though still facing challenges in broader adoption and specific indications.
The frontier of insulin delivery is undeniably defined by closed-loop artificial pancreas systems. By seamlessly integrating continuous glucose monitoring with intelligent algorithms and insulin pumps, these systems are ushering in an era of semi-automated glycemic management, demonstrably improving Time in Range, reducing hypoglycemia, and profoundly alleviating the immense mental burden of living with diabetes. Simultaneously, ongoing research into truly non-invasive methods, such as advanced transdermal patches and oral insulin, alongside the pervasive integration of artificial intelligence and machine learning, signals a future where diabetes management could become even more autonomous, personalized, and seamlessly integrated into daily life. (pmc.ncbi.nlm.nih.gov/articles/PMC11246106/)
Despite these profound advancements, challenges persist. High costs, ensuring equitable access, overcoming technological complexities, and addressing the psychosocial aspects of living with and managing a chronic condition remain critical areas requiring continued focus. The collaborative efforts of researchers, clinicians, engineers, and policymakers are crucial to surmount these hurdles. The continuous pursuit of more effective, safer, and more convenient insulin delivery methods is not merely an academic endeavor but a compassionate imperative, promising a future where individuals with diabetes can live healthier, fuller lives with significantly reduced disease burden and enhanced well-being.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
References
- en.wikipedia.org/wiki/Injector_pen
- en.wikipedia.org/wiki/Insulin_pump
- en.wikipedia.org/wiki/Smart_insulin_patch
- en.wikipedia.org/wiki/Automated_insulin_delivery_system
- iomcworld.com/open-access/transforming-diabetes-management-the-latest-in-insulin_delivery-systems-130856.html
- en.wikipedia.org/wiki/Dexcom_CGM
- jpionline.org/10.4103/2230-973x.176456
- biomedres.us/fulltexts/BJSTR.MS.ID.004161.php
- frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1566743/full
- pmc.ncbi.nlm.nih.gov/articles/PMC11246106/
- journals.lww.com/ijdt/fulltext/2023/02010/advancements_in_insulin_delivery_technology__a.5.aspx
- reuters.com/business/healthcare-pharmaceuticals/health-rounds-inhaled-insulin-good-injection-children-mealtime-2025-06-25/
- reuters.com/business/healthcare-pharmaceuticals/health-rounds-once-weekly-insulin-eli-lilly-appears-effective-daily-injections-2024-09-11/


Wow, a *comprehensive* report! Impressed by the smart insulin pen section – data-rich, indeed. So, when do *we* get chips that automatically order pizza when our blood sugar drops too low? Asking for a friend who may or may not be me.
Thanks for the kind words! The smart pen section was fascinating to research. About the pizza-ordering chip… that’s an interesting idea. Perhaps future AI could anticipate the low blood sugar *before* it happens and suggest a healthier snack alternative first? What do you think?
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
Fascinating deep dive! Regarding those AI-driven phenotypes: Will our insulin pumps soon know us better than we know ourselves? I, for one, welcome our new overlords…provided they keep my blood sugar stable.
Thanks for your comment! It’s a fascinating question, isn’t it? AI-driven phenotypes have the potential to revolutionize personalized medicine. Imagine pumps proactively adjusting to subtle changes in your body’s needs, far beyond what we can consciously track. That level of precision could significantly improve overall health outcomes.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe