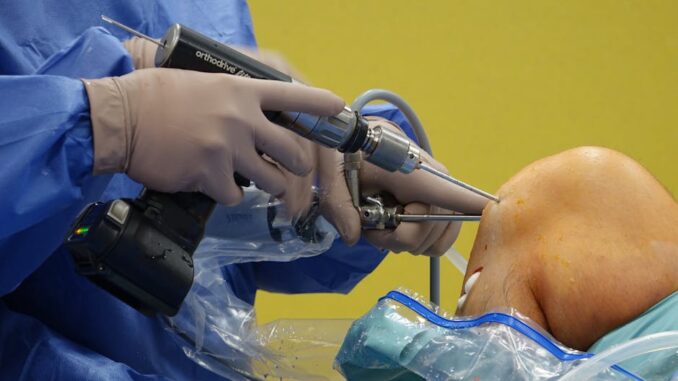
In a significant move to safeguard patient safety, the U.S. Food and Drug Administration (FDA) has announced a Class I recall—the most severe category—for certain Codman Disposable Perforators and Craniotomy Kits manufactured by Integra LifeSciences. This recall stems from a defect in the devices’ ultrasonic welds, which can lead to device disassembly during surgical procedures, posing serious risks to patients.
The Defect and Its Implications
The affected devices are designed to drill access holes into the skull during neurosurgical procedures. However, an inadequate ultrasonic weld on the outer sleeve of these devices may cause them to disassemble before, during, or after use. Such malfunctioning can result in the device becoming lodged in the patient’s skull, leading to complications like bleeding, brain injury, and extended surgical times. In some cases, the device may fail to disengage, preventing it from stopping immediately, which can cause irreversible brain damage or even death.
Safeguard patient information with TrueNASs self-healing data technology.
Reported Injuries
The FDA has received reports of 10 injuries associated with the use of these defective devices. These incidents include procedural delays, difficulty removing device fragments, bleeding, dural injury, and cerebral injury. Notably, there have been no reports of death linked to this issue. The injuries underscore the critical need for immediate action to prevent further harm to patients.
Recall Details and Manufacturer’s Response
Integra LifeSciences initiated the recall in April 2025 after identifying the defect. The company has urged healthcare providers to:
- Immediately stop using and quarantine all affected products.
- Review inventory and identify impacted lot numbers.
- Complete and return the appropriate Acknowledgement Form to Integra.
- Notify all relevant clinical or distribution staff.
- Return affected products after receiving a Return Material Authorization (RMA).
These steps aim to mitigate the risks associated with the defective devices and ensure patient safety.
Regulatory Classification and Safety Concerns
The FDA’s designation of this recall as Class I highlights the severity of the potential risks. Class I recalls are reserved for situations where there is a reasonable probability that the use of the product will cause serious adverse health consequences or death. This classification emphasizes the urgency for healthcare providers to act promptly to remove the affected devices from their inventories.
Broader Context of Medical Device Recalls
This incident is part of a broader pattern of medical device recalls aimed at addressing safety concerns. For instance, in February 2025, the FDA issued a Class I recall for certain external drainage and monitoring systems (EDMS) manufactured by Medtronic Neurosurgery, following 15 patient injuries linked to leaks and cracks in the devices. Such recalls underscore the ongoing challenges in ensuring the safety and reliability of medical devices used in critical procedures.
Recommendations for Healthcare Providers
Healthcare providers are strongly advised to:
- Immediately cease the use of the affected Codman Disposable Perforators and Craniotomy Kits.
- Quarantine all affected products to prevent accidental use.
- Review inventory to identify and segregate impacted lot numbers.
- Complete and return the Acknowledgement Form provided by Integra LifeSciences.
- Notify all relevant clinical and distribution staff about the recall.
- Arrange for the return of affected products after obtaining a Return Material Authorization (RMA) from Integra.
By taking these actions, healthcare providers can play a crucial role in preventing further patient injuries and ensuring the safety of surgical procedures.
Conclusion
The FDA’s recall of specific Codman Disposable Perforators and Craniotomy Kits serves as a stark reminder of the importance of rigorous quality control in medical device manufacturing. It also highlights the critical role of healthcare providers in promptly addressing recalls to protect patient safety. As the medical community continues to advance in technology, maintaining vigilance and adhering to safety protocols remain paramount to prevent adverse outcomes.


The reported injuries are concerning. What mechanisms are in place to ensure that hospitals and surgical centers are immediately notified of Class I recalls, and what verification processes confirm that affected devices are removed from use? This seems crucial to patient safety.
That’s a very important question! Beyond manufacturer notifications, hospitals often have internal risk management and patient safety departments that track recalls. Verification processes can include physical inspection, documentation checks, and internal audits. It’s a multi-layered approach, but always evolving. How can we improve post market surveillance?
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe