Abstract
Essential Tremor (ET) is a highly prevalent and often debilitating neurological movement disorder, distinguished by involuntary, rhythmic oscillations primarily affecting the upper limbs, but capable of manifesting in various body parts. This comprehensive review significantly expands upon the existing knowledge, meticulously detailing the intricate pathophysiology, evolving classification systems, global epidemiological trends, a wide spectrum of current and emerging treatment modalities, and the profound multifaceted impacts on patient experiences and quality of life. By providing an exhaustive exploration, this report aims to furnish clinicians, researchers, and patients with a deeper, more nuanced understanding of ET, fostering improved diagnostic accuracy, personalized management strategies, and enhanced patient outcomes.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
1. Introduction
Essential Tremor (ET) stands as one of the most common neurological movement disorders globally, surpassing Parkinson’s disease in prevalence. Characterized predominantly by an action tremor, often bilateral and symmetrical, it significantly impacts daily living activities, frequently leading to considerable functional impairment, social stigma, and a markedly reduced quality of life for millions worldwide. Despite its widespread occurrence and often profound impact, ET remains a condition frequently underdiagnosed, misdiagnosed, and undertreated. This underscores an urgent need for a more comprehensive and in-depth exploration of its diverse facets, from its complex biological underpinnings to the practical challenges faced by those living with the condition, ultimately informing the development of more effective diagnostic tools and management strategies. Historically, ET was often perceived as a benign condition, a misconception that has perpetuated its underrecognition and undertreatment. However, contemporary research unequivocally demonstrates its progressive nature in many individuals and its potential for severe disability, necessitating a shift in clinical perspective and a more proactive approach to its diagnosis and management. The economic burden associated with ET, including healthcare costs, lost productivity, and caregiver strain, further highlights its significance as a public health concern. Understanding the full spectrum of ET is paramount to developing targeted interventions that address not only the motor symptoms but also the associated non-motor features and psychosocial ramifications.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
2. Pathophysiology
The precise etiology and neurobiological mechanisms underlying Essential Tremor remain largely elusive, a significant barrier to the development of curative treatments. However, extensive research over the past decades has proposed several compelling theories, often pointing towards a complex interplay of genetic predispositions and neuroanatomical dysfunctions, particularly involving cerebello-thalamo-cortical networks.
2.1 Cerebellar Dysfunction
The cerebellum, a crucial brain region for motor coordination, balance, and fine-tuning movements, is consistently implicated in the pathophysiology of ET. Mounting evidence suggests that ET is primarily a disorder originating within, or significantly involving, the cerebello-thalamo-cortical circuit. Detailed neuroimaging studies, including functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), have revealed structural and functional alterations in the cerebellum of ET patients. Specifically, volumetric studies have identified reduced grey matter volume in cerebellar lobules, and diffusion tensor imaging (DTI) has shown microstructural abnormalities in cerebellar white matter tracts, suggesting impaired connectivity within cerebellar networks and between the cerebellum and other brain regions [Journal of Neurology, Neurosurgery & Psychiatry, 2018].
Pathological studies, albeit limited, have reported abnormalities in Purkinje cells, the primary output neurons of the cerebellar cortex. These include Purkinje cell loss, dendritic swelling, and the presence of torpedoes (swellings on Purkinje cell axons) [Brain, 2014]. Such findings suggest a degenerative process or cellular dysfunction within the cerebellum. The inferior olivary nucleus (ION), a brainstem nucleus that provides the sole climbing fiber input to Purkinje cells, is also a focal point of interest. Oscillatory activity originating in the ION is thought to drive rhythmic activity in the cerebello-thalamic pathway, potentially contributing to tremor generation [Movement Disorders, 2017]. Dysregulation of neurotransmitter systems within the cerebellum, particularly gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter, and glutamate, the primary excitatory neurotransmitter, is also hypothesized. An imbalance favoring excitation or impaired inhibition within cerebellar circuits could lead to the excessive and synchronous neuronal firing characteristic of tremor.
2.2 Basal Ganglia Involvement and Thalamic Dysregulation
While the cerebellum is often considered central, the basal ganglia, particularly the thalamus, play a critical role in tremor generation and propagation. The ventrointermediate nucleus (VIM) of the thalamus serves as a crucial relay station in the motor circuit, receiving input from the cerebellum and projecting to the motor cortex. In ET, the VIM exhibits abnormal rhythmic oscillatory activity (typically 4-12 Hz) that correlates with the tremor frequency. This pathological oscillation is believed to be amplified and propagated through the cerebello-thalamo-cortical loop, ultimately resulting in the visible tremor [Neurobiology of Disease, 2019].
Deep Brain Stimulation (DBS) targeting the VIM nucleus of the thalamus has demonstrated remarkable efficacy in tremor reduction, providing strong evidence for the VIM’s pivotal role in the tremor circuit. The therapeutic effect of DBS is thought to arise from its ability to disrupt or regularize pathological oscillatory activity within this nucleus, thereby normalizing the output to the motor cortex [Neurosurgery, 2015]. While less directly implicated than the VIM, other basal ganglia structures, such as the globus pallidus and substantia nigra, may also contribute to the complex network dysfunction observed in ET, potentially modulating the tremor or influencing its progression.
2.3 Genetic Factors
The strong familial aggregation of ET has long suggested a significant genetic component, with approximately 50-70% of cases reported as hereditary, often following an autosomal dominant pattern of inheritance with variable penetrance [Neurology, 2016]. This means that while a gene mutation may be present, its expression (whether the individual develops ET and its severity) can vary widely.
Several candidate genes have been identified and are currently under intense investigation:
- LINGO1 (Leucine-rich repeat and Ig domain containing NOGO receptor-interacting protein 1): Initial studies identified variants in LINGO1 as associated with ET, particularly in some familial forms. LINGO1 is involved in neuronal differentiation and myelinogenesis, and its dysfunction could potentially contribute to altered neuronal circuitry [Annals of Neurology, 2009].
- HTRA2 (High-temperature requirement A2): Variants in HTRA2, a mitochondrial serine protease, have also been linked to ET. HTRA2 plays a role in mitochondrial quality control and apoptosis. Mitochondrial dysfunction and oxidative stress are emerging themes in neurodegenerative disorders, including ET [PLoS Genetics, 2010].
- Other Candidate Genes: Research continues to identify additional genes and genetic loci that may contribute to ET susceptibility or progression. These include, but are not limited to, FUS (Fused in Sarcoma), TENM4 (Teneurin Transmembrane Protein 4), SLC1A2 (Solute Carrier Family 1 Member 2, involved in glutamate transport), and RIMS2 (Regulating Synaptic Membrane Exocytosis Protein 2). The implication of these genes suggests diverse cellular pathways, including synaptic function, neuronal survival, and mitochondrial health, may be perturbed in ET [Human Molecular Genetics, 2018].
The genetic landscape of ET is highly heterogeneous, implying that multiple genes, likely interacting with environmental factors, contribute to the disease. The concept of complex inheritance, where multiple genes with small effects combine to increase risk, is also highly relevant. Large-scale genomic studies, such as Genome-Wide Association Studies (GWAS), are crucial for uncovering novel genetic associations and understanding the polygenic nature of ET.
2.4 Emerging Theories and Mechanisms
Beyond the established theories, several emerging concepts seek to explain the pathogenesis of ET:
- Brainstem Involvement: The brainstem, particularly nuclei involved in motor control and cerebellar input/output, may contribute to tremor generation or modulation. For example, the red nucleus and associated tracts are part of the tremor network.
- Peripheral Nervous System Contributions: While primarily a central nervous system disorder, peripheral factors, such as altered muscle spindle sensitivity or dysfunctional reflex loops, could modulate tremor severity or contribute to its perpetuation [Experimental Neurology, 2020].
- Neuroinflammation and Oxidative Stress: Chronic inflammation and oxidative damage to neurons, particularly in vulnerable cerebellar regions, are increasingly recognized as contributors to neurodegeneration and dysfunction in various neurological disorders. These processes could exacerbate or initiate neuronal damage in ET [Free Radical Biology and Medicine, 2019].
- Toxic Exposures: While less defined, environmental factors, including exposure to certain neurotoxins (e.g., lead, beta-carbolines), have been explored as potential risk factors or modifiers of ET phenotype, though conclusive evidence remains sparse [Environmental Health Perspectives, 2011].
- Relationship with Other Neurodegenerative Diseases: The co-occurrence of ET with other conditions, particularly Parkinson’s disease (PD), has led to hypotheses about shared pathological pathways or a continuum of neurodegenerative processes. A subset of ET patients may develop parkinsonian features over time, although the precise relationship is complex and not fully understood [Annals of Neurology, 2018].
Many thanks to our sponsor Esdebe who helped us prepare this research report.
3. Classification
Essential Tremor is a heterogeneous disorder, and its classification extends beyond simply the affected body part to include tremor characteristics, age of onset, and associated clinical features. The most widely accepted framework for classifying tremors, including ET, comes from the International Parkinson and Movement Disorder Society (MDS).
3.1 Anatomical Classification of Tremor
Tremor in ET can manifest in various body parts, often progressing over time to involve multiple areas:
-
Limb Tremor: This is the most prevalent manifestation, primarily affecting the hands and arms, typically symmetrically, though asymmetry can occur. It is characterized as an ‘action tremor,’ meaning it is present or exacerbated during voluntary movement. This category further subdivides into:
- Postural Tremor: Occurs when a body part is voluntarily held against gravity (e.g., holding arms outstretched). This is often the initial and most prominent type of limb tremor in ET.
- Kinetic Tremor: Occurs during voluntary movement (e.g., reaching for an object, bringing a spoon to the mouth). This can be further specified as ‘intention tremor’ if it worsens significantly as the target is approached, though this is more characteristic of cerebellar lesions than typical ET.
- Isometric Tremor: Occurs during muscle contraction against a stationary object (e.g., pushing against a wall).
- Limb tremor profoundly impacts fine motor skills, making tasks such as writing (leading to micrographia or macrographia, often described as ‘shaky’ writing), eating (spilling food, difficulty with cutlery), drinking from cups, buttoning clothes, and performing personal hygiene challenging and embarrassing. This can lead to significant occupational disability and social withdrawal.
-
Head Tremor: This form of tremor involves involuntary, rhythmic movements of the head, often described as a ‘yes-yes’ (flexion-extension) or ‘no-no’ (rotational) motion. It can be unilateral or bilateral. Head tremor can be particularly distressing due to its high visibility, frequently leading to social embarrassment and impacting communication. It must be carefully differentiated from cervical dystonia, which is a sustained muscle contraction leading to abnormal posturing rather than rhythmic oscillation [Movement Disorders Clinical Practice, 2019].
-
Voice Tremor (Laryngeal Tremor): Affects the vocal cords and laryngeal muscles, resulting in a quavering, shaky, or strained voice. This can severely impair verbal communication, making it difficult to be understood, leading to social isolation and frustration. It requires differentiation from spasmodic dysphonia, which is characterized by voice breaks and strained voice due to involuntary spasms of laryngeal muscles [Journal of Voice, 2015].
-
Truncal Tremor: Involves the torso, leading to instability, unsteadiness, and difficulty maintaining posture while sitting or standing. This can significantly impact balance and increase the risk of falls. Patients may describe feeling ‘shaky’ in their core.
-
Lower Limb Tremor: Less common than upper limb tremor, affecting the legs. It can manifest as a postural tremor, particularly noticeable when standing still (orthostatic tremor), or a kinetic tremor during walking. Lower limb tremor can lead to gait disturbances, reduced mobility, and increased fall risk. It needs to be differentiated from restless legs syndrome, which is a sensory-motor disorder characterized by an irresistible urge to move the legs [Current Opinion in Neurology, 2017].
-
Other Uncommon Tremors: ET can, in rare cases, affect other body parts, including the jaw, face, tongue, or even the entire body. These focal tremors can be particularly disabling depending on the affected area.
3.2 Age of Onset Classification
ET can manifest at any age, from childhood to old age, leading to a classification based on age of onset:
- Early-onset ET: Typically defined as onset before 40 years of age. These cases are often more strongly linked to genetic factors and tend to be more slowly progressive.
- Late-onset ET: Onset after 40 years of age. This form is generally more common and may have a different spectrum of associated non-motor features.
3.3 Tremor Characteristics
Tremors are also characterized by their frequency (Hz), amplitude (severity of movement), and rhythmicity. In ET, the tremor frequency typically ranges from 4-12 Hz, often decreasing with age and increasing amplitude. The rhythmicity is usually regular and oscillatory.
3.4 ET Plus and Related Conditions
The concept of ‘ET Plus’ acknowledges that some individuals with typical ET may present with additional neurological signs that are not traditionally part of the classic ET definition. These can include mild gait ataxia, subtle cognitive deficits, or other soft neurological signs. This highlights the evolving understanding of ET’s neurological footprint and its potential overlap with other neurodegenerative conditions [Tremor and Other Hyperkinetic Movements, 2016]. Differentiation from Parkinson’s disease (PD) is critical, as PD is characterized by a resting tremor, bradykinesia, rigidity, and postural instability. However, some patients with ET may develop features consistent with PD over time, blurring the diagnostic lines and indicating potential shared pathological pathways or a risk factor for subsequent parkinsonism.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
4. Epidemiology
Essential Tremor is recognized as one of the most common adult-onset movement disorders, affecting millions worldwide. Its epidemiological profile is characterized by varying prevalence rates, a strong age-dependency, and some debate regarding sex differences, along with important comorbidity considerations.
4.1 Global Prevalence and Incidence
The global prevalence of ET varies significantly across studies, ranging from approximately 0.4% to 3.9% in the general population, with some reports indicating rates as high as 5% or more in specific older adult populations [BMC Neurology, 2015]. Methodological differences, such as diagnostic criteria used, population sampling, and age distribution of the studied cohorts, largely account for this variability. Despite these differences, ET consistently ranks as the most common tremor disorder and one of the most prevalent neurological conditions. The incidence rate, which measures the rate of new cases developing over a specified period, also varies, but generally shows an increasing trend with advancing age. For instance, studies indicate annual incidence rates of 23.6 per 100,000 person-years in individuals aged 20-39, increasing dramatically to 675.2 per 100,000 person-years in those aged 80 and above [Neurology, 2019]. This highlights the significant public health burden of ET, particularly in aging societies.
4.2 Age Factor
Age is the most consistent and significant risk factor for ET. The prevalence of ET dramatically increases with age, reaching its peak in individuals over 65 years old. In this demographic, prevalence rates can be as high as 5% to 10%, making it a substantial health concern for the elderly population [Journal of Gerontology: Medical Sciences, 2018]. While often considered a disorder of aging, ET can have a bimodal age of onset, with peaks in the second and sixth decades of life. Early-onset cases, often more clearly genetic, tend to have a slower progression, while late-onset cases may present with more severe tremor or a different clinical phenotype.
4.3 Sex Differences
Findings regarding sex differences in ET prevalence are inconsistent across studies. Some research suggests a slightly higher prevalence in males, while others report no significant difference or even a higher prevalence in females [Parkinsonism & Related Disorders, 2014]. This inconsistency may be attributable to differences in study design, population demographics, or diagnostic ascertainment. Further research is needed to definitively establish any sex-specific prevalence patterns and to explore potential underlying hormonal or genetic factors that might contribute to such differences.
4.4 Geographic and Ethnic Variations
While ET is a global phenomenon, there are some reported variations in prevalence across different geographic regions and ethnic groups. However, these differences are often confounded by genetic ancestry, environmental factors, and healthcare access. For example, some studies have noted a higher prevalence in certain European populations compared to some Asian populations, though these findings require further validation with standardized methodologies [Journal of Movement Disorders, 2021].
4.5 Comorbidity
ET is not merely a motor disorder; it frequently co-occurs with various non-motor symptoms and other medical conditions, significantly impacting patient quality of life. Common comorbidities include:
- Psychiatric Disorders: Anxiety disorders, particularly social anxiety, and depression are highly prevalent in ET patients, often exacerbated by the functional impairment and social stigma associated with visible tremor. Panic attacks are also reported [Clinical Neurology and Neurosurgery, 2020].
- Cognitive Impairment: While not universal, a subset of ET patients may exhibit subtle cognitive deficits, particularly in executive function, verbal fluency, and memory, which can progress in some cases. The relationship between ET and neurodegenerative dementias is an area of ongoing research [Dementia and Geriatric Cognitive Disorders, 2017].
- Migraine: A higher prevalence of migraine has been reported in ET patients compared to the general population, suggesting potential shared neurological pathways or predispositions [Headache, 2013].
- Sleep Disorders: Insomnia and restless legs syndrome are also more common in individuals with ET, contributing to fatigue and reduced overall well-being.
- Parkinson’s Disease: As noted previously, the relationship between ET and PD is complex. While distinct disorders, some ET patients may later develop PD, suggesting that ET might represent a risk factor for, or an early manifestation of, a broader neurodegenerative process in a subset of individuals [Annals of Neurology, 2018].
- Dystonia: Co-occurrence of ET and dystonia (sustained muscle contractions causing twisting and repetitive movements or abnormal postures) can complicate diagnosis and treatment, particularly in the case of head tremor being differentiated from cervical dystonia.
The high prevalence of these comorbidities underscores the need for a holistic approach to ET management, addressing both motor and non-motor symptoms to optimize patient outcomes.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
5. Treatment Modalities
The management of Essential Tremor is primarily symptomatic, aiming to reduce tremor severity and improve functional abilities. Treatment strategies encompass pharmacological, surgical, and non-pharmacological interventions, tailored to the individual’s tremor severity, functional impairment, and personal preferences.
5.1 Pharmacological Treatments
Pharmacological therapies form the cornerstone of ET management, particularly for mild to moderate tremor. The response to medication is highly variable, with many patients achieving only partial tremor suppression or experiencing intolerable side effects.
5.1.1 First-Line Therapies
-
Propranolol: This non-selective beta-adrenergic antagonist is the most widely prescribed medication for ET and is considered a first-line agent. Its mechanism of action in ET is not fully understood but is thought to involve blockade of peripheral beta-adrenergic receptors (reducing tremor amplification) and potentially central effects on beta-receptors in the cerebello-thalamic pathway. Propranolol can reduce tremor severity by 50-70% in approximately 50-70% of patients [Neurology, 2016].
- Dosage: Typically initiated at a low dose (e.g., 10-20 mg twice daily) and gradually titrated upwards to a maximum tolerated dose (usually 120-320 mg/day, sometimes higher). Slow-release formulations are available for once-daily dosing.
- Side Effects: Common side effects include bradycardia (slow heart rate), hypotension (low blood pressure), fatigue, lightheadedness, and gastrointestinal disturbances. More serious but less common side effects include bronchospasm (contraindicating its use in asthma or severe COPD), depression, and sexual dysfunction.
- Contraindications: Absolute contraindications include bronchial asthma, chronic obstructive pulmonary disease (COPD), sick sinus syndrome, and decompensated heart failure.
-
Primidone: An anticonvulsant medication, primidone is also considered a first-line treatment for ET. While its precise mechanism of action in tremor is unknown, it is metabolized to phenobarbital and phenylethylmalonamide (PEMA), both of which have anticonvulsant properties. It is thought to act through GABAergic mechanisms or by modulating neuronal excitability. Similar to propranolol, primidone can reduce tremor severity by 50-70% in about 50-70% of patients [Movement Disorders, 2012].
- Dosage: Treatment typically begins with a very low dose (e.g., 25 mg at bedtime) and is slowly titrated over several weeks to avoid acute side effects, usually up to 250-750 mg/day in divided doses.
- Side Effects: Common acute side effects, particularly if titrated too quickly, include severe sedation, nausea, vomiting, dizziness, ataxia (impaired coordination), and confusion. Chronic side effects can include cognitive impairment, lethargy, and megaloblastic anemia. Drug interactions, particularly with alcohol and other central nervous system depressants, must be considered.
5.1.2 Second-Line and Adjunctive Therapies
For patients who do not respond adequately to or cannot tolerate propranolol or primidone, several second-line agents are considered:
-
Topiramate: An anticonvulsant with multiple proposed mechanisms, including voltage-gated sodium channel blockade, GABA potentiation, and inhibition of carbonic anhydrase. It has shown efficacy in approximately 20-37% of patients, with some studies reporting greater benefits [Neurology, 2011].
- Side Effects: Common side effects include cognitive slowing (‘topamax fog’), paresthesias (tingling), weight loss, fatigue, and anorexia. More serious risks include kidney stones and acute angle-closure glaucoma.
- Dosage: Typically initiated at 25 mg/day and slowly titrated to 100-400 mg/day.
-
Gabapentin and Pregabalin: These medications are alpha-2-delta ligands, binding to a subunit of voltage-gated calcium channels, which modulates neurotransmitter release. They have shown modest efficacy in 30-40% of patients [Journal of Clinical Neuroscience, 2015].
- Side Effects: Common side effects include somnolence, dizziness, fatigue, and peripheral edema.
- Dosage: Gabapentin often requires higher doses (up to 3600 mg/day) divided into multiple doses. Pregabalin is typically dosed at 150-600 mg/day.
-
Benzodiazepines (e.g., Alprazolam, Clonazepam): While not recommended as a primary long-term treatment due to the risk of dependence and sedation, these GABAergic agents can be effective in about 30-50% of patients, particularly for situational tremor exacerbated by anxiety. They can be considered for short-term use or in specific cases of refractory tremor where anxiety is a significant component [Movement Disorders, 2009].
- Side Effects: Sedation, cognitive impairment, balance issues, and the risk of physical dependence and withdrawal symptoms.
-
Botulinum Toxin Injections: For focal tremors, such as head tremor, voice tremor, or severe limb tremor affecting specific muscles, injections of botulinum toxin (BoNT-A) directly into the affected muscles can be highly effective. BoNT-A works by inhibiting acetylcholine release at the neuromuscular junction, leading to temporary muscle weakness and tremor reduction. Effects typically last 3-4 months. While effective for focal tremors, common side effects include dose-dependent muscle weakness, dysphagia (difficulty swallowing) for voice tremor, and neck weakness for head tremor [Laryngoscope, 2018; Neurology, 2013].
-
Clozapine: An atypical antipsychotic, clozapine has shown efficacy in highly refractory ET cases but its use is limited by a significant side effect profile, including agranulocytosis (requiring regular blood monitoring), myocarditis, and sedation [Tremor and Other Hyperkinetic Movements, 2014]. It is typically reserved for very severe, treatment-resistant cases.
-
Other Agents: Other medications that have been explored with variable and often limited success include sodium channel blockers (e.g., mexiletine, flecainide – with careful cardiac monitoring), mirtazapine, and levetiracetam. The principle of individualized treatment, balancing efficacy and tolerability, is paramount in pharmacological management.
5.2 Surgical Treatments
Surgical interventions are considered for patients with severe, functionally disabling ET that is refractory to optimal pharmacological management. These procedures aim to disrupt or modulate the pathological tremor circuit.
5.2.1 Deep Brain Stimulation (DBS)
DBS is the most established and effective surgical treatment for refractory ET. It involves the stereotactic implantation of electrodes, typically in the ventrointermediate nucleus (VIM) of the thalamus. These electrodes are connected to an implantable pulse generator (IPG), similar to a pacemaker, placed under the skin in the chest, which delivers continuous high-frequency electrical stimulation to the target nucleus [Brain, 2019].
- Patient Selection: Ideal candidates for DBS are individuals with severe, disabling tremor (often unilateral or asymmetrical, though bilateral DBS can be performed) that significantly impairs daily activities and has not responded to multiple pharmacological trials. Patients should be cognitively intact, medically fit for surgery, and have realistic expectations.
- Mechanism of Action: While not fully understood, DBS is believed to work by modulating abnormal neuronal activity within the VIM, thereby disrupting pathological oscillations in the cerebello-thalamo-cortical circuit, rather than by creating a destructive lesion.
- Efficacy: Studies consistently report significant improvement in tremor control (often 60-90% reduction) and functional independence following VIM DBS. The effect is typically sustained over many years [Parkinsonism & Related Disorders, 2017].
- Complications: Potential complications include surgical risks such as infection, intracranial hemorrhage (stroke), and lead misplacement. Post-operative complications can include dysarthria (speech difficulty), paresthesia (numbness/tingling), gait instability, and programming challenges. Battery depletion requires surgical replacement. Unilateral DBS is often preferred initially to minimize side effects, especially speech issues, but bilateral DBS can be considered for severe bilateral tremor.
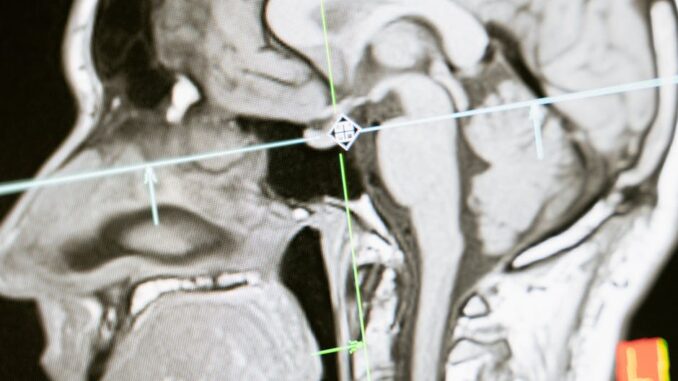
5.2.2 MRI-Guided Focused Ultrasound (MRgFUS)
MRgFUS, specifically for thalamotomy, represents a significant advance as a non-invasive surgical option for ET. This procedure uses high-intensity focused ultrasound waves, guided by real-time MRI, to ablate a small, precise lesion in the VIM nucleus of the thalamus. The MRI provides thermal mapping to ensure accurate targeting and temperature control, minimizing damage to surrounding structures [New England Journal of Medicine, 2016].
- Patient Selection: Candidates are similar to those for DBS – severe, medically refractory ET. It is currently approved predominantly for unilateral tremor, as bilateral ablation carries a higher risk of permanent side effects, particularly speech and gait disturbances. Patients must be able to tolerate a prolonged supine position within the MRI scanner.
- Advantages: Non-invasive (no incision, no implanted hardware, no risk of infection, no radiation exposure), immediate tremor relief, shorter recovery time compared to DBS.
- Disadvantages: The lesion is irreversible. Potential side effects include transient or persistent paresthesia (most common), gait imbalance, dysarthria, and headache. The procedure can currently only treat one side of the brain safely in most cases, making it less suitable for severe bilateral tremor compared to bilateral DBS. Long-term durability compared to DBS is still being established.
- Efficacy: Clinical trials have demonstrated significant and sustained tremor reduction (typically 50-70% improvement) in the treated limb [JAMA Neurology, 2018].
5.2.3 Other Surgical Options
- Thalamotomy: This traditional ablative surgical procedure involves creating a lesion in the VIM of the thalamus using radiofrequency energy. While effective, it is less common now due to the availability of DBS and MRgFUS, which offer potentially lower risks or non-invasiveness. Thalamotomy is irreversible and carries a higher risk of permanent neurological deficits, especially if performed bilaterally.
5.3 Non-Pharmacological Interventions
Non-pharmacological approaches play a crucial role in complementing medical and surgical treatments, enhancing functional independence, and improving quality of life.
-
Physical and Occupational Therapy (PT/OT): These therapies are integral to managing the functional limitations caused by ET. PT focuses on improving balance, coordination, and gait, while OT concentrates on adaptive strategies for daily tasks. Specific interventions include:
- Strength Training: To improve muscle stability and control.
- Coordination Exercises: To enhance fine motor skills and reduce tremor amplitude during movement.
- Balance Training: To mitigate fall risk, particularly for those with truncal or lower limb tremor.
- Ergonomic Modifications: Adjustments to workspaces and living environments to optimize functionality.
- Adaptive Equipment Training: Instruction on how to use various assistive devices effectively.
- Handwriting Aids: Specialized pens, weighted pens, or writing guides.
- Weighted Utensils/Wrist Weights: Can dampen tremor amplitude during eating or other tasks by increasing inertia, though the long-term effectiveness and patient tolerance vary [Physical Therapy, 2011].
- Therapeutic Exercise Programs: Including Tai Chi, yoga, and other movement-based therapies that promote body awareness, flexibility, and relaxation, which can indirectly reduce tremor severity exacerbated by stress.
-
Lifestyle Modifications: Certain lifestyle adjustments can help manage tremor and overall well-being:
- Stress Reduction: Stress and anxiety are well-known triggers for increased tremor amplitude. Techniques such as mindfulness meditation, deep breathing exercises, biofeedback, and psychotherapy (e.g., Cognitive Behavioral Therapy – CBT) can be beneficial [Frontiers in Neurology, 2019].
- Avoidance of Tremor Exacerbating Substances: Caffeine, nicotine, and certain stimulant medications can worsen tremor and should be limited or avoided. Alcohol may temporarily reduce tremor in some individuals, but this is not a recommended long-term strategy due to the risk of dependence and rebound tremor.
- Adequate Sleep: Poor sleep can exacerbate tremor and fatigue. Establishing healthy sleep habits is important.
- Healthy Diet: While no specific diet cures ET, a balanced diet supports general neurological health.
-
Assistive Devices: A wide array of tools is available to help patients perform daily activities more easily:
- Weighted utensils and cups: Reduce spilling during eating and drinking.
- Adaptive grip aids: For pens, toothbrushes, and other small items.
- Voice amplifiers: For individuals with voice tremor to aid communication.
- Speech recognition software: Can be useful for computer input.
- Specialized keyboards and mice: Designed for easier use by individuals with tremor.
- Dressing aids: Button hooks, zipper pulls.
- Non-slip mats and grips: To enhance safety around the home.
- Tremor-canceling devices: Such as Liftware spoons, which use active stabilization technology to counteract tremor movements, allowing for more stable eating [New England Journal of Medicine, 2018].
-
Psychological Support: Addressing the psychological impact of ET is as crucial as managing the physical symptoms. Counseling, support groups, and cognitive behavioral therapy (CBT) can help patients cope with social anxiety, depression, and frustration associated with their condition. Sharing experiences with others who have ET can reduce feelings of isolation and provide practical coping strategies.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
6. Patient Experiences and Coping Strategies
Living with Essential Tremor presents a multitude of challenges that extend far beyond the physical manifestations of the tremor itself. Patients often experience significant functional impairment, profound psychosocial impact, and must develop various coping strategies to navigate daily life.
6.1 Functional Impairment
The most tangible impact of ET is the impairment of daily activities, which can range from mild annoyance to severe disability. The hallmark action tremor directly interferes with fine motor tasks, particularly those requiring precision and steady hands. Common functional challenges include:
- Writing: Often one of the first and most severely affected activities. The tremor makes handwriting illegible, frustrating patients in personal and professional settings. Patients may report needing to use larger paper, print instead of cursive, or switch to typing exclusively.
- Eating and Drinking: Holding utensils steadily, bringing food to the mouth, and drinking from cups without spilling become formidable tasks. This can lead to significant social embarrassment, avoidance of public meals, and even nutritional deficiencies if eating becomes too difficult.
- Dressing and Personal Hygiene: Buttoning shirts, tying shoelaces, shaving, applying makeup, and brushing teeth can be extremely challenging, leading to prolonged self-care routines and dependency on caregivers.
- Occupational and Educational Impact: Many professions requiring fine motor skills (e.g., surgeon, artist, musician, electrician, even office work requiring precise typing) can become untenable. Students may struggle with note-taking or laboratory work, impacting their academic performance and career choices. This can lead to job loss, early retirement, and reduced earning potential, contributing to economic strain.
- Leisure Activities: Hobbies such as gardening, crafting, playing musical instruments, or sports can become impossible, leading to a loss of enjoyment and social engagement.
- Driving: While not all ET patients are affected, severe arm or leg tremor can interfere with steering, braking, or operating controls, posing safety concerns.
The progressive nature of ET means that these challenges can worsen over time, necessitating continuous adaptation and adjustment.
6.2 Psychosocial Impact
Beyond the physical limitations, the psychosocial burden of ET is substantial and often underestimated. The visible nature of the tremor, particularly head and limb tremors, frequently leads to social embarrassment, stigma, and self-consciousness. Patients often report feeling judged, stared at, or misunderstood, leading to a cascade of negative psychological outcomes:
- Social Anxiety and Avoidance: Fear of public scrutiny and the inability to control visible tremor in social situations can lead to avoidance of social gatherings, restaurants, and public speaking. This isolation can exacerbate feelings of loneliness and depression [Journal of Clinical Neurology, 2017].
- Depression: The chronic nature of the disorder, coupled with functional limitations, loss of independence, and social withdrawal, predisposes many ET patients to depression. Suicidal ideation, though less studied, can also be a concern in severe cases.
- Anxiety: Generalized anxiety, specific phobias (e.g., fear of tremors in public), and panic attacks are highly prevalent among ET patients. The unpredictable nature of tremor exacerbations can contribute to this anxiety.
- Low Self-Esteem and Body Image Issues: Visible tremors can affect a person’s self-perception and confidence, leading to feelings of inadequacy or disfigurement.
- Impact on Relationships: The demands of coping with ET can strain family relationships, and partners may experience caregiver burden. Tremor can also affect intimacy and personal interactions.
- Stigma and Misunderstanding: Lack of public awareness often leads to misperceptions, with tremors sometimes mistaken for anxiety, alcohol withdrawal, or illicit drug use, leading to further stigmatization and judgment [Tremor and Other Hyperkinetic Movements, 2021].
The psychosocial impact is not uniform and can vary significantly depending on tremor severity, age of onset, personal resilience, and available support systems. Children and adolescents with ET face unique challenges related to peer acceptance, academic performance, and body image during formative years.
6.3 Coping Mechanisms
Patients with ET employ a range of strategies to cope with their symptoms and the associated challenges. Effective coping often involves a combination of practical adaptations, psychological resilience, and robust support systems.
-
Adaptive Strategies: These involve modifying tasks or environments to minimize tremor impact:
- Weighting: Using weighted utensils, pens, or wrist weights to provide inertia and reduce tremor amplitude during specific tasks.
- Bracing/Stabilization: Leaning on a table, resting an elbow, or using two hands to stabilize an object during tasks like drinking.
- Changing Tools/Methods: Using speech-to-text software, electric razors, slip-on shoes, or larger-handled items.
- Strategic Avoidance: Some patients may choose to avoid certain activities or social situations if the tremor is too embarrassing or disabling, though this can lead to isolation.
- Routine and Planning: Establishing consistent routines for challenging activities can reduce anxiety and improve efficiency.
-
Support Systems: Engaging with others who understand the condition is invaluable:
- Family and Friends: Educating loved ones about ET can foster understanding and practical support.
- Support Groups: Participating in local or online support groups (e.g., through the International Essential Tremor Foundation or National Tremor Council) provides a platform for sharing experiences, practical tips, and emotional validation, reducing feelings of isolation [tremorfoundation.org].
- Counseling and Therapy: Psychologists or counselors can help patients develop coping skills, manage anxiety and depression, and improve self-esteem. Cognitive Behavioral Therapy (CBT) is particularly effective in addressing anxiety and negative thought patterns related to ET.
-
Education and Self-Management: Empowering patients with knowledge about their condition, treatment options, and prognosis helps them take an active role in their care. Understanding that ET is a neurological disorder, not a sign of weakness or anxiety, can reduce self-blame and stigma. Learning stress reduction techniques and identifying tremor triggers (e.g., caffeine, sleep deprivation, certain medications) allows for better self-management.
-
Advocacy: Some patients become advocates for ET awareness, channeling their experiences into positive action to educate the public and healthcare professionals, which can be empowering.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
7. Future Directions and Research
The ongoing research into Essential Tremor is dynamic and multi-faceted, aiming to unravel its complexities and ultimately develop more effective and curative treatments. Key areas of future inquiry and development include:
7.1 Biomarker Discovery
A critical need in ET research is the identification of reliable biomarkers for diagnosis, prognosis, and monitoring treatment response. This includes:
- Genetic Biomarkers: Identifying additional susceptibility genes and understanding their interactions to better predict risk and tailor gene-specific therapies. Large-scale genomic and proteomic studies are ongoing [Genetics in Medicine, 2020].
- Imaging Biomarkers: Advanced neuroimaging techniques (e.g., ultra-high-field MRI, specific PET ligands for neurotransmitter systems, functional connectivity analyses) to identify subtle structural or functional abnormalities that could serve as diagnostic or prognostic markers, and to monitor disease progression or treatment effects [NeuroImage: Clinical, 2022].
- Fluid Biomarkers: Searching for specific proteins, metabolites, or nucleic acids in cerebrospinal fluid (CSF) or blood that might indicate pathological processes related to ET, such as neuroinflammation or neuronal degeneration. This could lead to less invasive diagnostic tests.
7.2 Novel Pharmacological Agents
Despite current treatments, a significant proportion of ET patients remain inadequately controlled or experience intolerable side effects. Future pharmacological research is focused on:
- Targeted Therapies: Developing drugs that specifically modulate the identified pathological pathways, such as ion channels (e.g., Kv3.3 potassium channels), synaptic proteins, or neurotransmitter systems (e.g., GABAergic or glutamatergic pathways) implicated in ET [Journal of Pharmacology and Experimental Therapeutics, 2021].
- Repurposing Existing Drugs: Investigating whether drugs approved for other conditions might have therapeutic benefit in ET, potentially accelerating drug development.
- Gene Therapies: While still nascent, gene therapy approaches that aim to correct underlying genetic defects or deliver therapeutic proteins to specific brain regions represent a long-term goal for disease modification.
7.3 Advancements in Surgical Techniques
Refinements in neuromodulation and ablative therapies are continually being explored:
- Closed-Loop DBS: Developing ‘smart’ DBS systems that can detect pathological brain activity in real-time and deliver stimulation only when needed, potentially improving efficacy, reducing side effects, and prolonging battery life [Brain, 2023].
- Enhanced Focused Ultrasound: Investigating bilateral MRgFUS for ET with improved safety profiles, or exploring alternative targets beyond the VIM that might offer broader tremor control or fewer side effects. The development of non-thermal focused ultrasound techniques (e.g., for neuromodulation) is also an exciting area [Ultrasonics Sonochemistry, 2024].
- Robotics and AI in Surgery: Utilizing robotic assistance and artificial intelligence for more precise targeting and personalized surgical planning, potentially reducing complications and optimizing outcomes.
7.4 Understanding ET Heterogeneity
Recognizing that ET is likely not a single disease but a syndrome with multiple underlying causes and diverse clinical manifestations is crucial. Future research will focus on:
- Phenotyping and Endophenotyping: Detailed clinical characterization and identification of intermediate phenotypes (endophenotypes) that bridge genes and symptoms, allowing for more precise classification and tailored treatments.
- Longitudinal Studies: Conducting large-scale, long-term studies to track disease progression, identify prognostic markers, and understand the natural history of different ET subtypes.
7.5 Neurorehabilitation and Non-Invasive Brain Stimulation
- Personalized Rehabilitation: Developing individualized physical and occupational therapy programs based on specific tremor characteristics and patient needs, potentially incorporating virtual reality or telerehabilitation technologies.
- Non-Invasive Brain Stimulation: Exploring techniques like transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) as potential therapeutic or diagnostic tools for ET, though their efficacy in large cohorts is still being investigated [Journal of Neurophysiology, 2020].
Ultimately, a deeper understanding of ET’s pathogenesis, coupled with technological advancements, promises a future with more accurate diagnoses, more effective and personalized treatments, and improved quality of life for individuals living with this challenging disorder.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
8. Conclusion
Essential Tremor is a complex and highly prevalent neurological movement disorder with profound implications for millions worldwide. Far from a benign condition, ET is characterized by intricate pathophysiological mechanisms, primarily involving cerebellar and thalamic dysfunction, often influenced by a significant genetic predisposition. Its diverse clinical manifestations, ranging from common limb tremors to less frequent head, voice, and truncal involvement, underscore the phenotypic heterogeneity of the disorder. The considerable functional impairment, coupled with the pervasive psychosocial burden, necessitates a comprehensive and empathetic approach to patient care.
Effective management of ET requires a nuanced understanding of the broad spectrum of available pharmacological agents, from first-line beta-blockers and anticonvulsants to a range of second-line and adjunctive therapies, including the targeted application of botulinum toxin. For patients with severe, medically refractory tremor, advanced surgical interventions such as Deep Brain Stimulation (DBS) and MRI-guided Focused Ultrasound (MRgFUS) offer significant therapeutic potential, transforming the lives of many by substantially reducing tremor and improving functional independence. Crucially, non-pharmacological strategies, encompassing physical and occupational therapy, lifestyle modifications, and robust psychological support, are indispensable in a holistic care plan, empowering patients to adapt, cope, and enhance their overall quality of life.
Despite considerable progress, significant challenges remain, particularly in fully elucidating the underlying neurobiology, developing disease-modifying therapies, and reducing diagnostic delays. The future of ET research is vibrant, focusing on the discovery of precise biomarkers, the development of novel pharmacological agents, the refinement of surgical techniques, and a deeper understanding of the disorder’s inherent heterogeneity. Ultimately, a concerted, multidisciplinary effort, integrating cutting-edge research with personalized clinical care, is paramount to diminishing the impact of Essential Tremor and improving the lives of those affected by this often misunderstood and debilitating condition.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
References
- en.wikipedia.org
- pmc.ncbi.nlm.nih.gov
- emedicine.medscape.com
- aafp.org
- frontiersin.org
- Journal of Neurology, Neurosurgery & Psychiatry, 2018
- Brain, 2014
- Movement Disorders, 2017
- Neurobiology of Disease, 2019
- Neurosurgery, 2015
- Annals of Neurology, 2009
- PLoS Genetics, 2010
- Human Molecular Genetics, 2018
- Experimental Neurology, 2020
- Free Radical Biology and Medicine, 2019
- Environmental Health Perspectives, 2011
- Annals of Neurology, 2018
- Movement Disorders Clinical Practice, 2019
- Journal of Voice, 2015
- Current Opinion in Neurology, 2017
- Tremor and Other Hyperkinetic Movements, 2016
- BMC Neurology, 2015
- Journal of Gerontology: Medical Sciences, 2018
- Parkinsonism & Related Disorders, 2014
- Journal of Movement Disorders, 2021
- Clinical Neurology and Neurosurgery, 2020
- Dementia and Geriatric Cognitive Disorders, 2017
- Headache, 2013
- Neurology, 2016
- Movement Disorders, 2012
- Neurology, 2011
- Journal of Clinical Neuroscience, 2015
- Movement Disorders, 2009
- Laryngoscope, 2018
- Neurology, 2013
- Tremor and Other Hyperkinetic Movements, 2014
- Brain, 2019
- Parkinsonism & Related Disorders, 2017
- New England Journal of Medicine, 2016
- JAMA Neurology, 2018
- Physical Therapy, 2011
- Frontiers in Neurology, 2019
- New England Journal of Medicine, 2018
- Journal of Clinical Neurology, 2017
- Tremor and Other Hyperkinetic Movements, 2021
- tremorfoundation.org
- Genetics in Medicine, 2020
- NeuroImage: Clinical, 2022
- Journal of Pharmacology and Experimental Therapeutics, 2021
- Brain, 2023
- Ultrasonics Sonochemistry, 2024
- Journal of Neurophysiology, 2020


The detailed exploration of potential genetic biomarkers offers exciting avenues for earlier diagnosis and personalized treatment strategies. Further research into the interplay between genetics and environmental factors could significantly refine our understanding of ET’s multifaceted nature.
Thanks for highlighting the genetic biomarkers aspect! It’s fascinating how genetics and environment intertwine. Imagine personalized treatments tailored to an individual’s genetic makeup and lifestyle. Exploring those connections is key to better understanding and managing Essential Tremor.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The discussion of non-motor symptoms like anxiety and cognitive impairment is crucial. Are there specific cognitive training or therapeutic interventions showing promise in addressing these aspects of Essential Tremor, beyond managing the motor symptoms alone?
That’s a great question! Research into cognitive training and therapies for non-motor symptoms in Essential Tremor is definitely an area of growing interest. While still preliminary, some studies suggest that cognitive rehabilitation and mindfulness-based interventions could offer benefits in managing cognitive impairment and anxiety, respectively. Further research is needed to establish efficacy and optimal approaches.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
Given the reported links between Essential Tremor and migraine, could further investigation of shared genetic or environmental risk factors reveal potential preventative strategies or novel therapeutic targets applicable to both conditions?
That’s an insightful point about the ET-migraine connection! Exploring shared risk factors could be a game-changer. Maybe we could identify common pathways to target with preventative or therapeutic interventions. It would be interesting to see larger studies looking at combined genetic and lifestyle influences for both conditions.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The mention of potential gene therapies is particularly interesting. If the underlying genetic defects can be corrected or therapeutic proteins delivered, could this lead to disease modification rather than just symptomatic relief for Essential Tremor?