Abstract
The optimal timing of umbilical cord clamping (UCC) at birth has been a pivotal subject of extensive research and evolving clinical practice in contemporary neonatal care. This comprehensive research report meticulously examines the paradigm shift towards delayed umbilical cord clamping (DCC), advocating for a minimum delay of 60 seconds from birth, extending up to several minutes. It delves deeply into the intricate physiological mechanisms underpinning the substantial benefits of DCC, exploring its profound impact on neonatal hemodynamics, hematological status, and long-term developmental trajectories. The report undertakes a rigorous review of seminal and recent research studies, distinguishing findings across diverse populations, including full-term and preterm infants, and scrutinizing the evidence base that has reshaped international clinical guidelines. Furthermore, it addresses critical considerations for varying birth scenarios, including the management of complications, and explores the minimal yet significant implications for maternal health. Finally, the report identifies and analyzes the practical challenges inherent in the widespread implementation of DCC within diverse birthing environments, proposing strategies for effective integration into routine obstetric and neonatal care protocols.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
1. Introduction
The timing of umbilical cord clamping has undergone a significant re-evaluation in obstetric and neonatal practice over the past two decades. Historically, immediate umbilical cord clamping (ICC), typically within 15-30 seconds of birth, was the prevailing standard. This practice was largely adopted without robust scientific evidence, driven by perceived benefits such as expediting placental delivery, facilitating prompt neonatal resuscitation, and a perhaps unfounded concern regarding potential neonatal polycythemia or maternal hemorrhage. However, a growing body of evidence, derived from numerous well-designed research studies, has progressively challenged the rationale for ICC, advocating instead for delayed umbilical cord clamping (DCC).
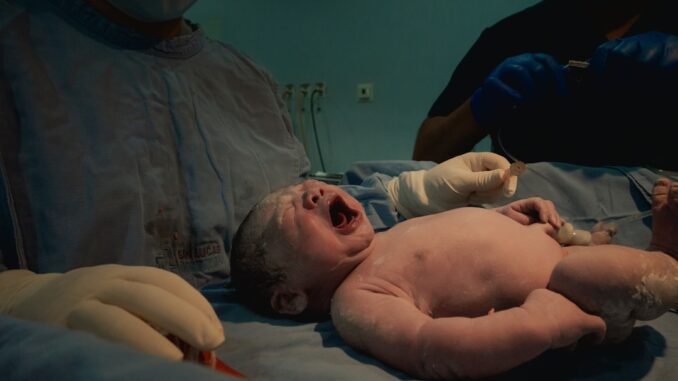
Delayed umbilical cord clamping refers to the practice of waiting for a specified period after birth – generally defined as 30 seconds to several minutes, or until umbilical cord pulsation ceases – before clamping and cutting the cord. This revised approach permits a continued physiological transfusion of placental blood to the newborn, a process rich in vital components crucial for neonatal adaptation to extrauterine life. The re-evaluation of this foundational perinatal practice represents a significant paradigm shift, promising substantial improvements in neonatal health outcomes across both full-term and, particularly, preterm populations. This report aims to provide an exhaustive analysis of the scientific rationale, clinical evidence, practical considerations, and challenges associated with the widespread adoption of DCC, underpinning its establishment as a standard of care endorsed by leading international professional organizations.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
2. Historical Practices and the Evolution of Umbilical Cord Clamping
The practice of umbilical cord clamping has a long and varied history, deeply intertwined with the evolution of obstetrics and neonatology. For centuries, the timing of cord clamping was often dictated by cultural practices or simply by the practicalities of birth, with many traditions favoring a delay. Ancient texts and traditional midwifery practices often describe waiting for the cessation of cord pulsation before clamping, implicitly recognizing the importance of the placental blood flow.
However, in the early 20th century, a shift towards immediate clamping began to emerge within Western medical practice. This transition was not primarily driven by empirical research demonstrating superiority but rather by a confluence of factors. One significant driver was the increasing medicalization of childbirth, where interventions and efficiency became prioritized. The ability to promptly attend to the infant and mother, particularly in scenarios of perceived distress or a need for rapid resuscitation, made immediate clamping seem advantageous. Early obstetric textbooks and medical teachings often recommended clamping within seconds of birth, sometimes citing concerns about potential ‘placental congestion’ or ‘over-transfusion’ leading to polycythemia in the infant, although these concerns were largely speculative at the time and lacked rigorous scientific validation.
Furthermore, the development of active management of the third stage of labor, aimed at reducing postpartum hemorrhage, sometimes included immediate cord clamping as part of a package of interventions (e.g., administration of oxytocin, controlled cord traction). While the efficacy of active management in reducing PPH is well-established, the specific role and necessity of immediate cord clamping within this framework were not definitively proven to contribute to the reduction of PPH and have since been largely decoupled in clinical recommendations.
Throughout the latter half of the 20th century, immediate clamping became the unquestioned norm in many parts of the world. It was only in the late 1990s and early 2000s that a resurgence of interest in delayed clamping began, fueled by pioneering research that systematically investigated its physiological impact. Researchers began to highlight the physiological continuity between the placenta and the newborn immediately after birth, likening the process to a ‘natural transfusion.’ This renewed scientific scrutiny revealed that the long-standing practice of immediate clamping might have inadvertently deprived newborns of a crucial physiological advantage, leading to a re-evaluation and ultimately a paradigm shift towards delayed clamping as the optimal approach.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
3. Physiological Mechanisms Behind the Benefits of Delayed Clamping
The benefits of delayed umbilical cord clamping are rooted in fundamental physiological processes that occur during the immediate postnatal period. Understanding these mechanisms is crucial for appreciating the profound impact of this simple intervention.
3.1. Placental Transfusion
At birth, a significant volume of blood, typically between 80 to 120 mL, remains within the placenta and umbilical cord. This blood is not simply residual but represents a vital reservoir intended for the neonate. Delayed clamping allows this ‘placental transfusion’ to continue, transferring approximately 30-50% of the placental blood volume to the infant over the first 1-3 minutes post-birth. The precise volume and rate of transfusion are influenced by several factors, including uterine contractions, gravity (infant positioning relative to the placenta), and the onset of neonatal respiration. As the infant takes its first breaths, pulmonary vascular resistance decreases, leading to increased blood flow to the lungs and a reduction in systemic pressure, which further facilitates blood flow from the placenta to the infant’s circulation.
3.2. Hematological Benefits
The additional blood volume received through DCC is rich in essential hematological components:
-
Increased Red Blood Cells and Hemoglobin: The most immediate and clinically significant benefit is the augmentation of the neonatal red blood cell mass. This translates to higher hemoglobin levels and improved hematocrit. For full-term infants, this increased iron endowment can significantly reduce the risk of iron-deficiency anemia in the first 6 to 12 months of life, a condition known to impair neurodevelopment. For preterm infants, the higher circulating red blood cell volume helps stabilize blood pressure and reduces the need for red blood cell transfusions, mitigating risks associated with transfusion, such as infection or circulatory overload.
-
Enhanced Iron Stores: The additional red blood cells deliver a substantial amount of iron to the newborn. Iron is a critical micronutrient vital for numerous physiological functions, including oxygen transport, energy metabolism, and neurological development. Adequate iron stores are essential for myelin formation, neurotransmitter synthesis, and overall brain growth and function. Preventing early iron deficiency through DCC can have long-lasting positive effects on cognitive, motor, and behavioral development.
3.3. Cardiopulmonary Transition and Hemodynamic Stability
The transition from fetal to neonatal circulation is one of the most dramatic physiological adjustments an individual undergoes. In utero, the placenta serves as the organ of gas exchange, and the lungs are largely inactive. Post-birth, the lungs must rapidly take over respiratory function, necessitating significant changes in cardiac output and vascular resistance. The additional blood volume from DCC plays a crucial role in this transition:
-
Improved Cardiac Output and Blood Pressure: The increased blood volume provides a more robust preload to the infant’s heart, supporting cardiac output and maintaining stable blood pressure. This is particularly vital for preterm infants, whose cardiovascular systems are less mature and more prone to instability.
-
Optimized Pulmonary Blood Flow: As the infant takes its first breaths, pulmonary arterioles dilate, allowing blood to flow into the lungs. The additional blood volume ensures adequate perfusion of the developing pulmonary vasculature, facilitating efficient gas exchange and reducing the risk of persistent pulmonary hypertension of the newborn.
3.4. Transfer of Stem Cells and Immunological Factors
Beyond red blood cells, placental blood is a rich source of other crucial biological components:
-
Stem Cells: Umbilical cord blood contains a high concentration of hematopoietic stem cells, which are critical for the development of the immune system and blood-forming tissues. It also contains mesenchymal stem cells and other progenitor cells. The transfer of these cells through DCC may contribute to tissue repair, anti-inflammatory processes, and neuroprotection, particularly relevant for vulnerable preterm infants who are at higher risk for conditions like intraventricular hemorrhage (IVH) and necrotizing enterocolitis (NEC).
-
Immunoglobulins and Clotting Factors: The additional blood also provides a boost of immunoglobulins, enhancing passive immunity, and various clotting factors, which can be beneficial in stabilizing the neonatal hemostatic system.
In essence, DCC allows for a more natural and complete physiological transition from intrauterine to extrauterine life, providing the newborn with a vital head start in adapting to its new environment. It leverages the inherent design of the fetal-placental unit to optimize neonatal health outcomes, moving away from an immediate separation that was once standard but is now recognized as potentially disadvantageous.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
4. Research Studies Supporting the New Guidelines
The shift towards delayed umbilical cord clamping is underpinned by a robust and continuously expanding body of scientific evidence, primarily derived from randomized controlled trials (RCTs), systematic reviews, and meta-analyses. These studies have meticulously compared outcomes between infants who received ICC and those who underwent DCC, providing compelling data that has informed contemporary clinical guidelines.
4.1. Research on Full-Term Infants
For full-term infants, the primary benefits of DCC are largely hematological and neurodevelopmental:
-
Prevention of Iron-Deficiency Anemia: Numerous studies have consistently demonstrated that DCC significantly improves iron status in healthy full-term infants. A landmark randomized clinical trial published in JAMA Pediatrics (Andersson et al., 2015) involving over 260 full-term infants found that delaying cord clamping for at least 3 minutes after birth resulted in significantly higher hemoglobin levels and reduced rates of iron deficiency at 4 months of age compared to infants who underwent early clamping (within 10 seconds). These benefits persisted, with follow-up studies showing improved iron stores and reduced anemia at 8 and 12 months of age. The World Health Organization (WHO) highlights that iron deficiency anemia is a major global health problem, impacting cognitive development, immune function, and physical growth. DCC, therefore, serves as a simple, cost-effective intervention to combat this widespread issue.
-
Neurodevelopmental Outcomes: The link between iron status and neurodevelopment has prompted investigations into the long-term cognitive and motor benefits of DCC. The same research group (Andersson et al., 2015) conducted a 4-year follow-up study, published in JAMA Pediatrics, on the children from their original RCT. They reported that children in the DCC group exhibited higher scores in fine-motor and social domains at four years of age, particularly among boys. This suggests that the improved iron endowment conferred by DCC may translate into tangible neurodevelopmental advantages, supporting optimal brain development during a critical period of growth. A study funded by the National Institutes of Health (NIH) also found that delayed cord clamping may benefit infant brain development, particularly in areas related to motor function, further reinforcing the long-term neurological advantages.
-
Risk of Polycythemia and Hyperbilirubinemia: Concerns about increased risks of polycythemia (abnormally high red blood cell count) and hyperbilirubinemia (jaundice) with DCC in full-term infants have been largely alleviated by research. While some studies have observed a statistically higher, though often clinically insignificant, incidence of mild polycythemia or a greater need for phototherapy for jaundice in DCC infants, these conditions are generally mild and easily managed. A meta-analysis by Fogarty et al. (2004) and subsequent reviews have concluded that any increase in hyperbilirubinemia requiring phototherapy is outweighed by the significant benefits of improved iron stores and reduced anemia, particularly in settings where iron deficiency is prevalent.
4.2. Research on Preterm Infants
For preterm infants, the benefits of DCC are even more profound and encompass a broader range of critical outcomes due to their inherent physiological vulnerabilities:
-
Improved Hemodynamic Stability and Blood Volume: Preterm infants often suffer from fragile circulatory systems and are prone to hypotension and poor tissue perfusion. DCC provides an essential additional blood volume, which helps stabilize blood pressure, improves cardiac output, and enhances cerebral perfusion. This improved hemodynamic stability can reduce the need for vasopressor support and expanders.
-
Reduced Need for Blood Transfusions: By increasing the infant’s own red blood cell mass at birth, DCC significantly reduces the need for subsequent blood transfusions in the neonatal intensive care unit (NICU). A meta-analysis by Rabe et al. (2012) found that DCC in preterm infants was associated with a lower incidence of anemia and a decreased requirement for red blood cell transfusions, thereby minimizing exposure to donor blood and its associated risks (e.g., transfusion-related acute lung injury, infection, volume overload).
-
Decreased Incidence of Intraventricular Hemorrhage (IVH): IVH, bleeding into the brain’s ventricular system, is a devastating complication common in preterm infants, leading to significant long-term neurological impairment. Several studies and meta-analyses, including a Cochrane review, have consistently demonstrated that DCC is associated with a significantly reduced incidence and severity of IVH, particularly Grade 3 and 4 hemorrhages. The proposed mechanism is that the increased blood volume and improved hemodynamic stability reduce fluctuations in cerebral blood flow, thus protecting the fragile cerebral capillaries from rupture.
-
Lower Rates of Necrotizing Enterocolitis (NEC): NEC, a life-threatening intestinal disease, is another major cause of morbidity and mortality in preterm infants. Research indicates that DCC is associated with a lower incidence of NEC. The enhanced placental transfusion likely improves gut perfusion and oxygenation, strengthens the intestinal barrier, and potentially transfers beneficial stem cells and immunological factors, all contributing to a healthier gastrointestinal tract in these vulnerable infants.
-
Reduced Incidence of Late-Onset Sepsis: Some studies suggest a lower incidence of late-onset sepsis in preterm infants who receive DCC. This could be attributed to the transfer of immunoglobulins and immune cells from the placenta, bolstering the immature immune system of preterm neonates.
-
Improved Survival Rates: A particularly compelling finding for preterm infants is the potential for improved survival. A study highlighted by Healthline in 2023 reported that delayed cord clamping could improve preterm infant survival rates by as much as 50%, underscoring the critical impact of this intervention in this vulnerable population.
-
Long-Term Neurodevelopmental Outcomes: While more research is ongoing, initial follow-up studies suggest that the protective effects of DCC against IVH and NEC may translate into better long-term neurodevelopmental outcomes for preterm infants, including improved cognitive and motor scores.
Collectively, these research findings have solidified the consensus among major professional organizations, including the American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics (AAP), and the World Health Organization (WHO), recommending delayed umbilical cord clamping as a standard practice for most births.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
5. Considerations for Different Birth Scenarios
The implementation of delayed umbilical cord clamping, while broadly beneficial, necessitates tailored considerations based on gestational age, mode of delivery, and the presence of maternal or fetal complications. A nuanced approach ensures that the benefits of DCC are maximized while mitigating any potential risks.
5.1. Preterm Infants
Premature infants, defined as those born before 37 weeks of gestation, are the population that often derive the most substantial and life-saving benefits from DCC. Their underdeveloped organ systems, particularly the cardiovascular and respiratory systems, make them highly susceptible to complications such as hypotension, intraventricular hemorrhage (IVH), and necrotizing enterocolitis (NEC).
-
Rationale: The additional blood volume and the supply of stem cells and immunological factors provided by DCC are critical for stabilizing the fragile circulatory system of preterm infants. This leads to improved blood pressure, better cerebral perfusion, and reduced requirements for blood transfusions and vasopressor medications. The protective effects against IVH and NEC are particularly important, directly impacting survival and long-term neurodevelopmental outcomes.
-
Implementation: For preterm infants, DCC should be prioritized whenever possible. This often requires adaptations in the delivery room. The infant should be kept at or below the level of the placenta during the delay period to facilitate placental transfusion. Maintaining normothermia is also crucial; this can be achieved by placing the infant skin-to-skin with the mother, wrapping the infant in warm towels, using plastic wrap, or employing a thermal mattress, especially during a cesarean section. If immediate resuscitation is anticipated but not actively required at birth, resuscitation efforts (e.g., tactile stimulation, airway clearance, positive pressure ventilation) can often be initiated while the cord remains intact, often referred to as ‘intact cord resuscitation’ or ‘physiologic cord clamping,’ to maximize the benefits of placental blood flow.
5.2. Full-Term Infants
Full-term infants (born at 37 weeks gestation or later) also benefit significantly from DCC, primarily through improved iron stores and reduced risk of iron-deficiency anemia, which has long-term neurodevelopmental implications.
-
Rationale: While the immediate life-saving benefits seen in preterm infants are less pronounced, the long-term advantages of preventing iron deficiency are significant for global child health. Adequate iron status in infancy is crucial for cognitive, motor, and behavioral development. DCC provides a natural, passive method of iron supplementation.
-
Implementation: For healthy full-term infants, DCC for at least 30-60 seconds, or longer (up to 3-5 minutes or until cord pulsation ceases), is recommended. This can be seamlessly integrated into routine care, often allowing for immediate skin-to-skin contact between mother and baby, which also promotes bonding, initiates breastfeeding, and aids in neonatal thermoregulation. Concerns about polycythemia or hyperbilirubinemia in full-term infants are generally mild and manageable, with the overall benefits far outweighing these transient risks.
5.3. Special Considerations and Complications
While DCC is the recommended standard for most births, there are specific clinical scenarios where immediate cord clamping (ICC) may still be necessary or preferred. These exceptions are typically guided by the need for immediate life-saving interventions for either the mother or the infant.
-
Maternal Hemorrhage/Instability: In cases of severe maternal hemorrhage, such as that caused by placenta previa, placental abruption, or uterine rupture, where maternal hemodynamic stability is compromised, immediate clamping may be necessary to allow for rapid treatment of the mother, even if it means sacrificing the benefits of DCC for the infant. However, for routine postpartum hemorrhage management, DCC is generally not found to increase the risk of PPH and is often compatible with active management of the third stage of labor (e.g., oxytocin administration before cord clamping).
-
Severe Fetal Distress Requiring Immediate Resuscitation: If an infant is born in severe distress, requiring immediate and aggressive resuscitation (e.g., prolonged bradycardia, apnea, or very low Apgar scores necessitating ventilatory support beyond simple stimulation), ICC may be chosen to facilitate rapid transfer to a resuscitation platform. However, the concept of ‘intact cord resuscitation’ is gaining traction, where some initial resuscitative steps can be performed while the cord remains attached, especially for preterm infants. This decision requires careful clinical judgment based on the severity of distress and available resources.
-
Placental Abruption: In cases of significant placental abruption, where there is a premature separation of the placenta from the uterus, the infant may have already experienced considerable blood loss in utero. While the principle of allowing continued placental transfusion is still relevant, the clinical urgency to assess and potentially resuscitate a compromised infant, coupled with the potential for ongoing maternal hemorrhage, often dictates ICC.
-
Certain Fetal Anomalies or Conditions: In rare instances, specific fetal conditions or anomalies, such as severe hydrops fetalis or known congenital heart disease requiring immediate intervention, may warrant ICC to facilitate prompt access for specialized care.
-
Multiple Gestations: For twins or higher-order multiples, DCC can still be attempted for each infant sequentially. However, care must be taken to prevent twin-to-twin transfusion syndrome if the babies share a placenta and the cord of the first twin is clamped too late or too early relative to the second twin. Protocols often involve clamping the first twin’s cord after a delay, followed by clamping the second, if conditions permit.
-
Cord Prolapse: While uncommon, if a cord prolapse necessitates an emergency delivery, the focus is on rapid extraction of the fetus, and DCC may be impractical or contraindicated due to the urgency.
It is imperative that healthcare providers possess the knowledge and clinical judgment to make informed decisions regarding cord clamping timing, always prioritizing the safety and well-being of both mother and infant while adhering to evidence-based guidelines.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
6. Impact on Maternal Health
One of the initial concerns regarding the widespread adoption of delayed umbilical cord clamping centered on its potential impact on maternal health, specifically the risk of postpartum hemorrhage (PPH). Historically, immediate cord clamping was sometimes considered a component of active management of the third stage of labor (AMTSL), a set of interventions aimed at reducing PPH. However, extensive research has largely allayed these fears, demonstrating that DCC has minimal, if any, adverse effects on maternal health and can be safely integrated with AMTSL.
6.1. Postpartum Hemorrhage Risk
Multiple randomized controlled trials and systematic reviews have investigated the relationship between DCC and the incidence of PPH. A comprehensive Cochrane review (McDonald et al., 2013) and subsequent meta-analyses consistently found no statistically significant increase in the risk of severe postpartum hemorrhage (defined as blood loss >1000 mL) or maternal blood transfusion requirements with delayed cord clamping compared to immediate clamping. Some studies have noted a slight, often clinically insignificant, increase in average blood loss in the DCC group (typically less than 60 mL), but this has not translated into an increased incidence of clinical PPH or adverse maternal outcomes.
This reassurance stems from understanding the physiological mechanisms controlling uterine contraction and placental separation. The primary drivers of uterine involution and prevention of PPH are effective uterine contractions (often facilitated by oxytocin administration) and prompt placental expulsion. While the timing of cord clamping does influence the volume of blood in the placenta, it does not directly impede the uterus’s ability to contract effectively or the placenta’s natural detachment process. In fact, many guidelines recommend administering oxytocin concurrently with DCC or immediately after birth, which effectively mitigates any theoretical increase in PPH risk.
6.2. Maternal Anxiety and Stress
Beyond the physiological aspects, the adoption of DCC can have positive indirect effects on maternal well-being. Knowing that their newborn is receiving optimal care and benefiting from a natural physiological process can reduce maternal anxiety and stress. The ability to facilitate immediate skin-to-skin contact during the DCC period also promotes early maternal-infant bonding, which has documented benefits for both mother and baby, including successful breastfeeding initiation and improved emotional well-being for the mother. This uninterrupted first contact contributes to a more positive birthing experience and can empower mothers in their early parenting journey.
6.3. Practical Considerations for Maternal Care
Implementing DCC requires minor adjustments to maternal care protocols but generally does not complicate them. For instance, the administration of prophylactic oxytocin for PPH prevention can occur during the delay period or immediately after the cord is clamped. Similarly, initial maternal assessment, such as checking for tears or lacerations, can often proceed during the DCC period. The focus remains on providing comprehensive and holistic care to both mother and infant, integrating DCC as a beneficial component of this care.
In summary, the evidence strongly supports that delayed umbilical cord clamping is a safe practice for mothers, with no significant increase in the risk of postpartum hemorrhage or other adverse maternal outcomes. This finding is critical for ensuring broad acceptance and implementation of DCC as a standard of care, without compromising maternal safety.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
7. Practical Implementation Challenges and Solutions
The successful integration of delayed umbilical cord clamping into routine obstetric and neonatal practice, while evidence-based and highly beneficial, is not without its challenges. These challenges span clinical, logistical, educational, and cultural domains, requiring thoughtful planning and concerted effort for effective implementation.
7.1. Clinical Protocols and Staff Training
- Challenge: Many healthcare providers, particularly those trained under older protocols, may be accustomed to immediate clamping and may lack familiarity with the updated guidelines and the physiological rationale behind DCC. There can be hesitation or discomfort with deviating from long-established routines. Ensuring consistent practice across all shifts and personnel is also difficult.
- Solution: Comprehensive, multidisciplinary training programs are essential. These should involve obstetricians, midwives, neonatologists, nurses, and residents. Training should cover the evidence base, the physiological benefits, practical techniques for DCC in various scenarios (vaginal birth, cesarean section, preterm), and clear identification of contraindications. Simulation exercises, scenario-based learning, and hands-on workshops can build confidence and competence. Regular audits and feedback loops are necessary to ensure adherence and identify areas for further training.
7.2. Infrastructure and Equipment Adaptations
- Challenge: Traditional delivery room setups often place the resuscitation bay some distance from the birthing mother, making DCC challenging, particularly for infants requiring any immediate assessment or minimal intervention. Maintaining the infant’s temperature during DCC, especially for preterm infants, also requires special attention.
- Solution: Birthing environments may need redesigning to allow for resuscitation to occur at the mother’s bedside while the cord remains intact. This might involve mobile resuscitation carts that can be brought close to the mother, adjustable radiant warmers, or specialized equipment to maintain warmth during skin-to-skin contact. For cesarean sections, adapting surgical drapes to create a ‘window’ or carefully positioning the infant on the mother’s abdomen immediately after delivery can facilitate DCC while maintaining a sterile field. The use of plastic wraps, warm blankets, or thermal mattresses can prevent hypothermia during the delay.
7.3. Time Management and Workflow Integration
- Challenge: The immediate postpartum period is often a busy and time-sensitive phase, with various tasks needing attention (e.g., Apgar scoring, maternal assessment, placental delivery, initial maternal-infant bonding, breastfeeding initiation). Integrating a 30-60 second or longer delay can feel disruptive to established workflows.
- Solution: Clinical protocols should clearly define the roles and responsibilities of the care team during DCC. For healthy infants, the DCC period can be seamlessly integrated with early skin-to-skin contact, which fulfills multiple beneficial roles simultaneously (thermoregulation, bonding, breastfeeding cues). Apgar scores can be assessed at 1 and 5 minutes, aligning well with a DCC protocol. For infants requiring more immediate assessment but not active resuscitation, initial checks can often be performed with the cord intact. Open communication and coordination among the birth team are paramount to ensure smooth transitions.
7.4. Cultural and Institutional Resistance
- Challenge: Resistance to change, deeply entrenched beliefs, and a lack of familiarity with new practices can hinder implementation. Some practitioners or institutions may be hesitant to adopt DCC due to perceived risks or simply a preference for the ‘way things have always been done.’ In some settings, the pressure to maintain a rapid workflow in high-volume birth centers can also be a barrier.
- Solution: Strong leadership and advocacy from key opinion leaders (e.g., department chairs, chief medical officers) are crucial. Disseminating up-to-date evidence through grand rounds, journal clubs, and internal communications can help overcome skepticism. Developing clear, evidence-based institutional policies and clinical practice guidelines, endorsed by senior leadership, provides a framework for consistent adoption. Highlighting successful implementation stories and the positive impact on patient outcomes can also foster a culture of acceptance.
7.5. Communication with Parents
- Challenge: Parents may not be aware of DCC or its benefits. If they have preconceived notions about immediate clamping or are anxious about the birth process, a deviation from what they expect could cause concern.
- Solution: Antenatal education classes and discussions with obstetric providers should include information about DCC, its benefits, and what to expect during birth. Providing clear, concise, and accessible information can empower parents to make informed decisions and feel more engaged in their birth experience. Explaining the ‘why’ behind the practice can alleviate anxieties and build trust.
7.6. Monitoring for Hyperbilirubinemia
- Challenge: While the benefits of DCC generally outweigh the risks, a slight increase in the need for phototherapy for hyperbilirubinemia in DCC infants has been noted in some studies. This requires vigilant postnatal monitoring.
- Solution: Routine postnatal screening for jaundice, including transcutaneous bilirubin measurements or serum bilirubin levels, should be standard practice for all newborns, including those who underwent DCC. Clear institutional protocols for managing hyperbilirubinemia, including indications for phototherapy, should be in place. Parent education on signs of jaundice and when to seek medical advice is also important.
By proactively addressing these challenges with thoughtful strategies, healthcare systems can successfully integrate delayed umbilical cord clamping into standard practice, ensuring that more newborns benefit from this simple yet profoundly impactful intervention.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
8. Ethical and Global Health Perspectives
8.1. Ethical Imperative
From an ethical standpoint, the widespread adoption of delayed umbilical cord clamping aligns with the principle of beneficence – acting in the best interest of the patient. Given the robust evidence demonstrating clear and significant benefits for newborns, particularly preterm infants, and the minimal to no harm to the mother, withholding DCC without specific medical contraindications could be viewed as suboptimal care. The intervention is non-invasive, cost-effective, and leverages natural physiological processes, making it an ethically sound practice to promote. It reflects a commitment to optimizing neonatal health outcomes based on the most current scientific understanding.
8.2. Global Health Impact
Delayed umbilical cord clamping holds particular significance in the context of global health, especially in low-resource settings where maternal and child mortality rates are higher, and access to advanced medical interventions is limited.
-
Combating Anemia: Iron deficiency anemia is a pervasive problem globally, affecting a significant proportion of children in low- and middle-income countries. This condition impairs cognitive development, reduces physical work capacity, and increases susceptibility to infections. DCC, by naturally boosting infant iron stores, offers a simple and highly cost-effective public health intervention to combat infant anemia without requiring expensive supplements or complex healthcare infrastructure. The World Health Organization (WHO) explicitly recommends DCC for all births in their guidelines for optimal cord clamping for the prevention of iron deficiency anemia in infants.
-
Improved Outcomes for Preterm Infants: Preterm birth is a leading cause of neonatal mortality worldwide. In settings with limited access to neonatal intensive care, basic interventions that improve preterm survival and reduce complications are invaluable. DCC, with its proven benefits in reducing IVH, NEC, and the need for transfusions, can dramatically improve the chances of survival and healthy development for preterm infants in these vulnerable populations.
-
Feasibility and Scalability: Unlike many complex medical interventions, DCC requires no specialized equipment or significant financial investment. It is a change in practice rather than an addition of technology, making it highly feasible to implement even in the most resource-constrained environments. This scalability makes it a powerful tool for improving neonatal health on a global scale, contributing to the achievement of Sustainable Development Goals related to maternal and child health.
-
Equity in Care: Promoting DCC helps reduce health disparities by ensuring that all newborns, regardless of their birthplace or socioeconomic status, have access to an intervention that significantly enhances their health and developmental trajectory.
However, implementing DCC in some global health contexts may still face challenges such as traditional practices, lack of awareness among healthcare providers, or overwhelming workloads in understaffed facilities. Therefore, targeted education, clear national guidelines, and community engagement are crucial for successful global implementation.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
9. Conclusion
The re-evaluation and subsequent widespread adoption of delayed umbilical cord clamping represent a significant triumph of evidence-based medicine in neonatal care. The journey from immediate clamping as a default practice to DCC as the recommended standard highlights the critical importance of continuous scientific inquiry and the willingness to challenge long-standing traditions in favor of optimal patient outcomes.
The physiological mechanisms underlying the benefits of DCC are now well-understood: it facilitates a crucial placental transfusion, enriching the newborn’s blood volume with vital red blood cells, iron, and stem cells. This physiological ‘boost’ is instrumental in supporting the complex cardiopulmonary transition to extrauterine life, improving hematological status, and potentially offering long-term neurodevelopmental advantages. The robust body of research unequivocally supports these benefits, with particularly profound impacts observed in vulnerable preterm infants, where DCC significantly reduces the incidence of life-threatening complications such as intraventricular hemorrhage and necrotizing enterocolitis, and even improves survival rates.
While tailored considerations are necessary for specific birth scenarios and the rare instances of maternal or fetal compromise may necessitate immediate clamping, the overwhelming evidence confirms DCC as safe and beneficial for the vast majority of newborns. Critically, concerns regarding adverse maternal outcomes, particularly postpartum hemorrhage, have been largely unsubstantiated by research. The challenges associated with implementing DCC, such as updating clinical protocols, reconfiguring birth environments, and overcoming institutional resistance, are surmountable through dedicated education, staff training, and strong leadership.
As DCC becomes the global standard of care, its impact resonates beyond individual births, offering a simple yet powerful public health intervention, particularly in combating infant anemia in low-resource settings and promoting equitable health outcomes worldwide. Continued research, especially in long-term neurodevelopmental follow-up, will further refine our understanding and reinforce the practice. Ultimately, delayed umbilical cord clamping stands as a testament to the power of physiological medicine and its potential to profoundly enhance the health and developmental trajectory of every newborn.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
References
- American Academy of Pediatrics. (2017). Timing of Umbilical Cord Clamping After Birth. Pediatrics, 139(6), e20171650. aap.org
- American College of Obstetricians and Gynecologists. (2016). ACOG Recommends Delayed Umbilical Cord Clamping for All Healthy Infants. Retrieved from acog.org
- American College of Obstetricians and Gynecologists. (2020). Delayed Umbilical Cord Clamping After Birth. Committee Opinion No. 684. Obstetrics & Gynecology, 136(4), e100–e101. acog.org
- Andersson, O., et al. (2015). Effect of Delayed Cord Clamping on Neurodevelopment at 4 Years of Age: A Randomized Clinical Trial. JAMA Pediatrics, 169(7), 631-638. jamanetwork.com
- Andersson, O., et al. (2019). Delayed Cord Clamping and Brain Development in Preterm Infants. The Lancet Child & Adolescent Health, 3(10), 733-740.
- Fogarty, M., et al. (2004). Delayed Cord Clamping for Term Babies: Systematic Review. BMJ, 329(7457), 24-25.
- Healthline. (2023). Delayed Cord Clamping Improves Preterm Infant Survival Rates by 50%, Study Finds. Retrieved from healthline.com
- Mayo Clinic Health System. (n.d.). Delayed Umbilical Cord Clamping – Benefits. Retrieved from mayoclinichealthsystem.org
- McDonald, S.J., et al. (2013). Effect of Timing of Umbilical Cord Clamping of Term Infants on Maternal and Neonatal Outcomes. Cochrane Database of Systematic Reviews, (7), CD004074.
- National Institutes of Health. (2019). Science Update: Delayed Cord Clamping May Benefit Infant Brain Development, NIH-Funded Study Finds. Retrieved from nichd.nih.gov
- Rabe, H., et al. (2012). Effect of Delayed Cord Clamping on Clinical Outcomes of Preterm Infants: A Systematic Review and Meta-Analysis. The Journal of Pediatrics, 161(2), 240-244.e1.
- Texas Children’s Hospital. (n.d.). Here’s Why Delayed Cord Clamping is the New Normal. Retrieved from texaschildrens.org
- World Health Organization. (2023). Optimal Timing of Cord Clamping for the Prevention of Iron Deficiency Anaemia in Infants. Retrieved from who.int


Be the first to comment