Summary
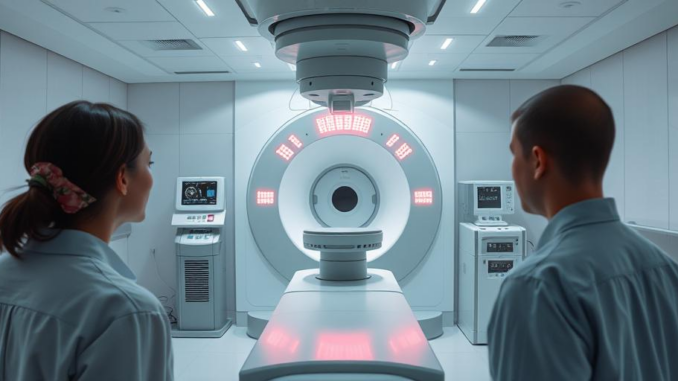
The FDA has cleared ZEISS’s INTRABEAM 700, a robotic-assisted radiation therapy platform for neurooncology and breast cancer. This innovative system offers enhanced precision, streamlined workflows, and seamless connectivity, potentially revolutionizing cancer treatment. The platform’s advanced features promise improved outcomes and a more efficient surgical experience.
Secure patient data with ease. See how TrueNAS offers self-healing data protection.
** Main Story**
Hey everyone, I wanted to share some exciting news from ZEISS Medical Technology. They’ve just received FDA 510(k) clearance for their INTRABEAM 700 platform, and it’s a real game-changer for intraoperative radiation therapy (IORT). This tech basically brings robotic precision to cancer treatment, particularly for neurooncological and breast cancer cases, and it really does look promising!
Think about it: we’re talking about enhanced precision, streamlined workflows, and seamless connectivity, all working together to make surgery more efficient and effective. It’s a huge step forward in cancer care.
Robotic Precision: Hitting the Target
The heart of this system is the SMART Stand, that’s the robotic part. It’s designed for incredible maneuverability and control, which means doctors can target tumors with unprecedented accuracy. It minimizes the impact on healthy tissue, which, as you know, is absolutely critical. The SMART Stand also has active damping to reduce vibrations, ensuring radiation is delivered consistently and accurately. This kind of precision can really maximize the treatment’s effectiveness and, importantly, cut down on complications.
I saw a demo last year, and the stability was really impressive. It gives surgeons that extra level of confidence when they’re delivering radiation. Remember that time our department had to deal with a similar issue with a previous machine? This promises to prevent that headache.
SMART Workflow: Making Things Easier
ZEISS has clearly thought about workflow efficiency too. The INTRABEAM 700 has a digital-first design that simplifies things. For instance, the SMART Spherical Applicators are sterile and single-use and are even color-coded. Plus, there’s a Spherical Sizer Set to help you pick the right applicator size. Also, you get digital-assisted applicator management, so no more sterilization hassles. The graphical interface is really intuitive, making the whole system much easier to use. Less time fiddling with equipment, more time focusing on the patient – that’s the idea.
These enhancements are designed to reduce procedural time, minimize disruptions, and allow healthcare professionals to focus on delivering the best possible patient care.
Seamless Connectivity: Working Together
The INTRABEAM 700 integrates smoothly into existing hospital systems, which is a big plus. This connectivity speeds up workflows and simplifies data management. The system even uses RFID to recognize and confirm applicators, which further boosts efficiency and accuracy. This interoperability promotes collaboration between neurosurgery and oncology teams, contributing to a more cohesive and effective treatment approach. And it supports remote access to treatment data, so you can monitor and optimize treatment protocols from afar.
I think that’s really important. How often do we struggle with getting different systems to talk to each other? This seems to address that head-on.
Radiance Software: Planning for Success
The Radiance treatment planning software is another cool feature. It allows clinicians to simulate radiation dose parameters preoperatively using patient-specific data. This helps minimize surprises during surgery and makes the treatment process more predictable. And I think that’s key for improved patient outcomes, don’t you? The software provides valuable insights for optimizing treatment strategies too.
The Future of IORT?
So, where does this leave us? The INTRABEAM 700 looks like a significant leap forward for IORT. It promises to improve treatment precision, streamline workflows, and enhance the surgical experience overall. It’s currently being used in clinical studies for brain tumors and breast cancer, and the initial results are pretty promising.
ZEISS is committed to innovation, and I think the INTRABEAM 700 could play a big role in shaping the future of cancer treatment. As more clinical data comes out, this could become the new standard of care for intraoperative radiation therapy. It’s definitely something to keep an eye on.
What do you think? Could this be the turning point we’ve been waiting for in cancer treatment? I, for one, am cautiously optimistic. It’s April 11, 2025, and this FDA clearance is definitely exciting news for medical technology and, hopefully, for better cancer care.


The integration of RFID for applicator recognition is intriguing; could you elaborate on how this system mitigates the risk of incorrect applicator selection and its impact on patient safety protocols?
That’s a great question! The RFID system indeed plays a crucial role in safety. It cross-checks the selected applicator against the planned treatment parameters, triggering an alert if there’s a mismatch. This helps prevent errors and ensures the correct radiation dosage is delivered, improving patient safety significantly. Let’s discuss further!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
Given the system’s connectivity, how does the platform ensure patient data privacy and security during remote access for treatment monitoring and optimization?
That’s a really important point! The INTRABEAM 700 incorporates advanced encryption and access controls to protect patient data during remote access. It also adheres to strict HIPAA compliance. We are working on an article that will describe the multi layered protection approach, to offer a more in depth description of data security.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe