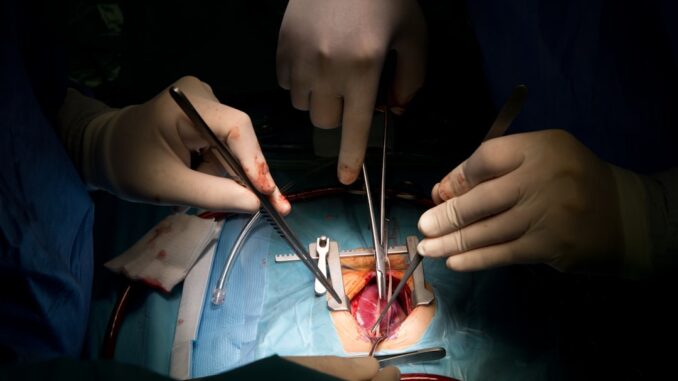
A New Beat: Revolutionizing Pediatric Heart Transplants
In the realm of pediatric heart transplantation, where every tick of the clock holds immense significance, a single donor heart truly possesses the power to transform not just one, but multiple young lives. It’s a field brimming with both profound hope and relentless challenge, but recent, rapid advancements in medical technology, coupled with genuinely collaborative efforts, have begun reshaping this landscape entirely. We’re seeing significantly improved outcomes for these incredibly brave young patients, and it’s nothing short of inspiring.
This isn’t just about better surgery; it’s a holistic revolution. From how we keep a heart viable during transit to the almost futuristic ways we match donors and recipients, and even the intricate, long-term care that follows, innovation is everywhere. You know, for families whose children face life-threatening cardiac conditions, these breakthroughs aren’t just scientific triumphs, they’re lifelines. Let’s delve into how these crucial innovations are literally giving children new beats and new futures.
Secure patient data with ease. See how TrueNAS offers self-healing data protection.
Breathing New Life into Organ Preservation and Transport
For decades, the standard procedure for transporting donor hearts felt almost antiquated. Surgeons would simply cool the organ down, pack it in ice, and rush it to the recipient, hoping against hope the limited cold ischemic time wouldn’t compromise its viability. This method, while groundbreaking in its time, severely constrained the window for successful transplantation, often limiting the practical travel distance for donor hearts. Think about it, a ticking clock, every minute counts, and if the donor was too far away, that precious organ might just be unusable.
Thankfully, we’ve moved beyond that. The advent of ex-vivo heart perfusion technology, often affectionately nicknamed ‘heart in a box’ technology, has fundamentally changed this paradigm. This isn’t just a fancy cooler; it’s a sophisticated machine that perfuses the heart with oxygenated blood, maintaining it in a warm, beating state outside the body during transit. It’s like having an intensive care unit on wheels for the organ itself, ensuring its metabolic needs are met.
The ‘Heart in a Box’: Expanding Horizons
This incredible system extends the traditional ischemic window from a mere 4-6 hours, sometimes to upwards of 10-12 hours or even longer. What does this mean in practical terms? It means a dramatically expanded donor pool. We can now consider hearts from donors located much further away, and crucially, even those previously deemed unsuitable due to what would have been extended cold ischemic times.
Consider the groundbreaking work at Mayo Clinic Children’s Center, for instance. They successfully performed their first pediatric transplant using this very technology, illustrating its immediate, tangible impact. This wasn’t just a procedural milestone; it opened up possibilities for young patients who might have waited even longer or never received a suitable organ. It’s about access, plain and simple, and for children, that’s everything. Imagine the relief for parents, knowing their child now has a better chance, simply because a heart can travel further and arrive in better condition.
And it’s not just about the distance. Maintaining the heart in a beating state allows for real-time assessment of its function before implantation. Surgeons can observe its performance, ensuring it’s robust and ready for its new home, which ultimately leads to better outcomes post-transplant. It’s a significant leap forward, don’t you think?
Donors After Circulatory Death (DCD) and On-Table Reanimation
Beyond the ‘heart in a box,’ another pivotal advancement lies in the utilization of hearts from Donors After Circulatory Death (DCD). Traditionally, donor hearts came from brain-dead donors whose hearts were still beating. DCD donation involves organs from patients whose hearts have stopped beating permanently. This pool, if tapped effectively, offers a substantial increase in available organs. However, using DCD hearts for transplantation presents unique challenges, particularly the period of warm ischemia after circulatory arrest but before organ retrieval.
This is where innovative techniques, like the one pioneered by researchers at Duke University, become absolutely critical. They’ve developed a novel method to assess heart function on a sterile operating table using what they call ‘on-table heart reanimation.’ After the heart is retrieved from a DCD donor, they carefully reperfuse and oxygenate it right there in the operating room. This allows surgeons to visually and functionally evaluate the heart’s viability and recovery potential.
For pediatric patients, this is a game-changer. The scarcity of appropriately sized and matched donor hearts for infants and young children is a persistent, heartbreaking challenge. Expanding the donor pool through DCD hearts, rigorously assessed with methods like on-table reanimation, means more chances, and potentially, significantly reduced wait times. Duke’s research suggests this technique could increase infant heart transplants by as much as 20%, which is, honestly, an astounding figure when you consider the stakes. It’s about giving every potential heart a fair shot, and every child a fighting chance.
Artificial Intelligence: The Smart Matchmaker
The journey from a donor heart to a recipient isn’t just about preserving the organ; it’s also about finding the perfect match. This process, historically, has been incredibly complex, labor-intensive, and time-sensitive. But today, Artificial Intelligence (AI) is stepping in, playing a truly pivotal role in enhancing the efficiency and accuracy of donor-recipient matching, particularly in the delicate world of pediatric cardiology.
Think of it as the ultimate matchmaker, not for romance, but for life itself.
Deep Learning for Precision Matching
At Cincinnati Children’s Hospital, a dedicated team of cardiologists and researchers is leveraging cutting-edge deep learning algorithms to analyze cardiac images with unparalleled speed and precision. In pediatric heart transplantation, one of the critical parameters for a successful match is the anatomical compatibility between the donor heart and the recipient’s chest cavity. Specifically, measuring the Total Cardiac Volume (TCV) has been a crucial, yet often time-consuming, aspect of this assessment.
Traditionally, this involved manual measurements, taking up precious minutes, or even hours, during a critical decision-making window. Now, AI-driven approaches can process and analyze these complex cardiac images – MRI scans, CT scans – almost instantaneously. The algorithms can accurately calculate TCV, assess ventricular dimensions, and even identify subtle anatomical variations that might impact surgical fit, all in a fraction of the time a human expert would require. This significantly reduces the time from donor offer to acceptance or rejection, streamlining the entire process.
Beyond Speed: Optimizing Outcomes
But the benefits of AI extend far beyond mere speed. By improving the precision of these anatomical matches, AI contributes directly to better transplant outcomes. A well-matched heart means less stress on the new organ, potentially fewer surgical complications, and a smoother recovery for the child. Furthermore, AI has the potential to integrate a vast array of other critical data points – immunological factors, blood types, genetic markers, and even predictive analytics on post-transplant success rates based on historical data. Imagine an algorithm sifting through thousands of past cases to predict the best possible outcome for a current child. That’s the power we’re talking about.
We’re only scratching the surface, you know. Future AI applications could involve predicting and preventing organ rejection by analyzing microscopic changes in biopsies, or even tailoring personalized immunosuppression regimens, minimizing side effects while maximizing organ longevity. The ethical considerations around AI, such as data privacy and algorithmic bias, are certainly important to monitor, but the potential for saving and improving lives here is truly immense. It’s not just about getting a heart; it’s about getting the right heart, and AI is making that more possible every day.
A United Front: Collaborative Efforts and Advocacy
The complex, multifaceted nature of pediatric heart transplantation demands more than individual brilliance; it requires a collective, concerted effort. No single institution or researcher can tackle the myriad challenges alone, especially when dealing with such a vulnerable patient population. That’s why the recent formation of the Pediatric Heart Transplant Alliance (PHTA) marks such a significant and encouraging milestone in the field. It’s a clear signal that the community is recognizing the power of unity.
The Power of Alliance
This formidable coalition brings together some of the most influential organizations in pediatric heart transplantation, including Enduring Hearts, the International Society for Heart and Lung Transplantation (ISHLT), and the Pediatric Heart Transplant Society (PHTS). Each of these entities brings unique strengths to the table, and by uniting them, the alliance creates a synergy that promises to accelerate progress on multiple fronts.
The alliance isn’t just a talking shop, mind you. Its core mission is ambitiously focused on advancing research, enhancing education, and strengthening advocacy in pediatric heart transplantation. What does that really mean?
- Research: They’re pooling resources and expertise to identify critical research gaps, fund innovative studies, and facilitate multi-center clinical trials. This could involve anything from developing new anti-rejection medications tailored for children to exploring novel genetic therapies or even bio-engineered organs. Imagine the combined brain power focused on one goal. It’s inspiring, frankly.
- Education: Ensuring that medical professionals, from surgeons and cardiologists to nurses and social workers, have access to the latest best practices, training, and knowledge is paramount. The PHTA will likely develop standardized guidelines, host educational symposia, and create platforms for knowledge exchange, ultimately elevating the standard of care across the board.
- Advocacy: This is where the alliance can truly amplify the voices of pediatric transplant patients and their families. They’ll advocate for increased public awareness about organ donation, especially pediatric donation, push for policy changes that support patient access to care, and champion funding for critical research. It’s about ensuring these children aren’t forgotten, that their unique needs are met, and that the system works more effectively for them.
By uniting these diverse stakeholders, the PHTA aims not only to improve transplant success rates but also to expand access to care for more children and, crucially, enhance the long-term quality of life for pediatric transplant recipients. It’s a holistic approach, recognizing that the journey doesn’t end with a successful surgery, it simply begins.
The Ripple Effect: Success Stories and Profound Impact
The true measure of these scientific and collaborative advancements, of course, isn’t found in a laboratory or a policy paper. It’s etched in the lives of the young patients and their families who have walked this arduous path. The impact is profound, stretching far beyond the operating room, touching every aspect of a child’s life and the hopes of their loved ones.
Think about Maya, a vibrant young girl whose story perfectly encapsulates the transformative power of these medical innovations. Before her transplant, Maya, like many children with severe heart conditions, likely faced limitations, fatigue, and perhaps even a fear of truly being a child. But shortly after her life-saving surgery, the transformation was remarkable. She was up and moving, her spirited personality, once perhaps dimmed by illness, shining brightly. Her recovery wasn’t just about surviving; it was about thriving. It underscores the incredible potential for pediatric heart transplant recipients to lead not just active, but truly fulfilling lives post-transplant, chasing dreams that once seemed utterly out of reach.
Similarly, Tenlee’s journey illuminates the profound emotional and physical metamorphosis that a new heart can bring. Imagine being a child unable to play, unable to run, watching friends from the sidelines. That’s often the reality for children awaiting a heart. After receiving her new heart, Tenlee’s health improved dramatically. The energy she gained allowed her to finally engage in activities she had previously been unable to enjoy, to truly experience childhood without the crushing burden of heart failure. Her story isn’t just about a medical procedure; it’s about reclaiming joy, independence, and a future that had once seemed uncertain.
These anecdotes aren’t mere feel-good stories; they are powerful testaments to the dedicated work of countless professionals and the incredible resilience of the human spirit. They remind us why this work matters so much, why the relentless pursuit of innovation is not just academic, but deeply human. Every successful transplant isn’t just a medical victory; it’s a child’s laughter, a parent’s sigh of relief, and a family’s renewed hope.
The Unseen Battle: Post-Transplant Care and Lifelong Vigilance
While the focus often understandably falls on the dramatic moments of transplantation – the donor match, the surgery itself – the true, long-term success story of pediatric heart transplantation is written in the meticulous, lifelong journey of post-transplant care. This phase is arguably as complex and critical as the surgery, requiring unwavering vigilance, a highly specialized medical team, and immense resilience from both the child and their family.
The Immunosuppression Tightrope
One of the most immediate and critical challenges post-transplant is managing the body’s natural immune response. The recipient’s immune system will naturally view the new heart as a foreign invader and attempt to reject it. To prevent this, children receive powerful immunosuppressant medications, often a cocktail of several drugs. This is a delicate balancing act, a tightrope walk, if you will.
- Preventing Rejection: Too little immunosuppression, and the body could reject the organ, leading to severe complications or even transplant failure. Acute rejection episodes, while less common with modern drugs, can still occur and require immediate, aggressive treatment.
- Minimizing Side Effects: Too much immunosuppression, and the child becomes highly vulnerable to opportunistic infections, which can be life-threatening. These medications also carry a host of potential long-term side effects, including kidney dysfunction, high blood pressure, diabetes, and an increased risk of certain cancers, particularly lymphomas.
Regular blood tests, biopsies, and careful monitoring are essential to adjust medication dosages, trying to find that perfect therapeutic window. For parents, this often means becoming amateur pharmacists, meticulously managing complex medication schedules and observing for even the slightest signs of trouble. It’s a constant, silent battle, fought daily in homes around the world.
Beyond the Heart: Holistic Care
Post-transplant care extends far beyond just the heart itself. These children require comprehensive, multidisciplinary support that addresses every facet of their well-being:
- Infection Control: Due to immunosuppression, common childhood illnesses can become serious threats. Families learn stringent hygiene practices and often have to limit exposure to crowds, especially during flu season.
- Growth and Development: For infants and young children, ensuring normal growth and neurological development despite chronic medication and potential illness is a priority. Nutritionists, physical therapists, and developmental specialists play crucial roles.
- Psychosocial Support: The emotional toll on the child and their family cannot be overstated. Anxiety, depression, PTSD, and the constant stress of potential complications are very real. Child psychologists, social workers, and peer support groups become vital resources, helping families navigate the emotional rollercoaster. Imagine the constant worry parents carry, even years after a successful transplant.
- Transition to Adult Care: As these children grow into adolescents and then young adults, the process of transitioning from pediatric to adult transplant care is another critical step. This involves empowering young patients to take ownership of their health management, understanding their complex medical history, and finding adult specialists who can provide continuity of care. It’s not always a smooth transition, and some patients can fall through the cracks if not carefully guided.
This continuous, comprehensive care is what allows transplant recipients to truly flourish. It’s a testament to the dedication of their medical teams and, most importantly, the incredible strength and commitment of the families who support them every single day.
Looking Ahead: The Future of Pediatric Heart Transplantation
The future of pediatric heart transplantation, while still grappling with formidable challenges, is undoubtedly promising. The relentless pursuit of innovation, driven by both necessity and ingenuity, continues to push the boundaries of what’s possible, promising even better outcomes and a significantly enhanced quality of life for young patients.
Overcoming Persistent Challenges
Despite the remarkable progress, the field faces ongoing hurdles. The fundamental challenge remains the severe scarcity of suitable donor organs, particularly for infants who often require hearts of specific sizes and blood types. This scarcity leads to agonizingly long wait times, during which a child’s condition can unfortunately deteriorate significantly.
Another major challenge is chronic rejection, also known as cardiac allograft vasculopathy (CAV). Even with optimal immunosuppression, the coronary arteries of the transplanted heart can slowly narrow over time, leading to heart failure years after the initial surgery. It’s a stealthy, persistent threat that can require re-transplantation, which carries even higher risks. So, preventing or reversing CAV is a critical area of ongoing research.
Emerging Horizons
Yet, the ingenuity of medical science knows no bounds, and several exciting avenues hold immense promise:
- Xenotransplantation: The idea of using animal organs, particularly from genetically modified pigs, for human transplantation is no longer science fiction. Recent breakthroughs in adult heart xenotransplantation, while still in very early stages, offer a tantalizing glimpse into a future where organ scarcity might be drastically reduced. Imagine what this could mean for a baby urgently needing a tiny heart.
- Regenerative Medicine and Bioengineering: Could we one day grow new hearts in a lab? Researchers are exploring decellularization techniques, where existing hearts are stripped of their cells, leaving only a scaffold, which can then be repopulated with a patient’s own stem cells. This would theoretically eliminate the need for immunosuppression entirely. While still nascent, the potential is revolutionary.
- Further AI Integration: We’ve discussed AI in matching, but its role will only expand. AI could be instrumental in predicting rejection episodes before symptoms appear, optimizing drug dosages in real-time, or even assisting surgeons with intra-operative guidance using augmented reality, leading to even more precise procedures.
- Advanced Imaging and Diagnostics: Continual improvements in non-invasive imaging techniques could allow for earlier detection of rejection or complications, enabling quicker interventions and preventing irreversible damage.
The integration of sophisticated AI, enhanced organ preservation techniques, and truly collaborative efforts among medical institutions, advocacy groups, and innovative biotech companies are paving the way for more successful transplants and, crucially, a much better quality of life for young patients. As these innovations continue to evolve and coalesce, the hope swells that more children will have the opportunity to receive a new heart, not just a chance at life, but a full, vibrant, and active lease on life. It’s a future we’re all, quite literally, rooting for.
References
-
Mayo Clinic Children’s Center specialists perform center’s first pediatric transplant with ‘heart in a box’ technology. mayoclinic.org
-
New Technique Could Increase Infant Heart Transplant by 20%. corporate.dukehealth.org
-
Improving Pediatric Heart Transplantation With Artificial Intelligence. childrenshospitals.org
-
Six Leading Organizations Unite to Launch the Pediatric Heart Transplant Alliance. ishlt.org
-
Heart Transplant: Maya’s Story. hopkinsmedicine.org
-
Heart Transplant: Tenlee’s Story. chop.edu


The use of AI to improve matching precision at Cincinnati Children’s Hospital is fascinating. Could these algorithms eventually predict long-term outcomes based on subtle genetic markers or even lifestyle factors, allowing for preemptive interventions and personalized post-transplant care plans?
That’s a fantastic point! The potential for AI to predict long-term outcomes and tailor post-transplant care is truly exciting. Imagine personalized immunosuppression regimens based on individual genetic profiles! It could revolutionize how we manage transplant patients. Thanks for sparking that thought!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The discussion on AI-driven matching is particularly compelling. Beyond anatomical compatibility, how might AI enhance long-term monitoring of pediatric patients, predicting potential complications like rejection through continuous analysis of physiological data and potentially preempting invasive procedures like biopsies?
That’s a really insightful question! AI’s potential for preemptive complication detection through continuous data analysis is immense. Imagine AI flagging subtle physiological changes indicative of early rejection, enabling personalized interventions and reducing the need for invasive procedures. This could truly revolutionize long-term care! #MedTech #AI
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe