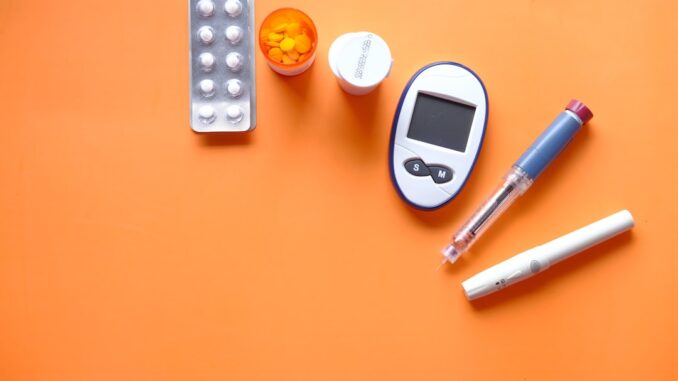
Abstract
Type 2 diabetes (T2D) represents a formidable global health challenge, characterized by its chronic, progressive nature and its significant impact on individual well-being and healthcare systems. The last decade has witnessed a profound transformation in T2D management, driven by rapid advancements in medical technology and digital health solutions. This comprehensive report undertakes an in-depth exploration of these innovative tools and systems, meticulously examining their design, operational principles, and multifaceted implications for patient care. Key innovations analyzed include Continuous Glucose Monitoring (CGM) systems, which provide dynamic glucose profiles; Automated Insulin Delivery (AID) systems, aiming to mimic physiological insulin regulation; smart insulin pens, enhancing adherence and data capture; and a diverse array of digital health platforms fostering self-management and remote care. Furthermore, the report delves into cutting-edge emerging technologies such as non-invasive glucose monitoring, sophisticated AI-driven predictive analytics, and highly personalized feedback systems. This analysis critically evaluates their demonstrated impact on critical patient outcomes, including glycemic control and complication rates, alongside improvements in quality of life, enhancements in healthcare efficiency, and considerations of accessibility. The report also scrutinizes the inherent challenges associated with the vast volumes of data generated, the complexities of their interpretation, and the intricate process of integrating these advanced solutions into established clinical workflows and broader healthcare infrastructures.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
1. Introduction
Type 2 diabetes, a metabolic disorder primarily characterized by insulin resistance and relative insulin deficiency, affects an estimated 537 million adults globally, with projections indicating a rise to 783 million by 2045 according to the International Diabetes Federation (IDF Diabetes Atlas, 10th edition). The escalating prevalence of T2D places an immense burden on individuals, healthcare providers, and national economies. Traditionally, T2D management has centered on foundational lifestyle interventions – encompassing dietary modifications and increased physical activity – coupled with a stepped approach to oral pharmacotherapy and, for many, eventual insulin therapy. While these strategies have been effective in mitigating some aspects of the disease, they often necessitate rigorous self-management, frequent clinical visits, and a significant cognitive load for patients attempting to maintain optimal glycemic control.
The inherent challenges in traditional diabetes care stem from several factors: the episodic nature of glucose measurements (e.g., fingerstick blood glucose monitoring provides only snapshots), the variability of individual responses to interventions, the difficulty in maintaining consistent adherence to complex treatment regimens, and the reactive rather than proactive nature of management. These limitations frequently lead to suboptimal glycemic control, increasing the risk of both acute complications (hypoglycemia, hyperglycemia) and long-term macrovascular (cardiovascular disease, stroke) and microvascular complications (retinopathy, nephropathy, neuropathy), ultimately diminishing quality of life and imposing substantial healthcare costs.
The advent of technological innovation has catalyzed a paradigm shift in diabetes care, moving towards more proactive, personalized, and data-driven approaches. This report aims to provide a detailed, evidence-based review of the transformative technologies that are reshaping T2D management. We will dissect the mechanisms, benefits, and challenges of established and emerging technologies, offering a comprehensive understanding of their current and future roles in improving patient outcomes, enhancing quality of life, and optimizing healthcare delivery. By thoroughly examining Continuous Glucose Monitoring (CGM) systems, Automated Insulin Delivery (AID) systems, smart insulin pens, digital health platforms, and other pioneering solutions, this report seeks to illuminate the profound impact these innovations are having on the global fight against Type 2 diabetes.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
2. Continuous Glucose Monitoring (CGM) Systems
2.1 Overview of CGM Technology
Continuous Glucose Monitoring systems represent a significant leap forward from traditional self-monitoring of blood glucose (SMBG) via fingersticks. Instead of discrete snapshots, CGMs provide a dynamic, near real-time picture of glucose fluctuations throughout the day and night. At their core, CGM systems consist of three primary components: a small, disposable sensor, a transmitter, and a receiver or compatible smart device (e.g., smartphone, smartwatch). The sensor, typically a thin filament, is painlessly inserted into the interstitial fluid, usually in the abdomen or upper arm, where it can remain for 10-14 days depending on the model. This sensor is coated with an enzyme, glucose oxidase, which reacts with glucose in the interstitial fluid to produce an electrical signal. The transmitter, affixed to the sensor, converts this signal into a glucose reading and wirelessly sends it to a receiver or a paired smart device every 1 to 5 minutes.
There are broadly two types of CGM systems: real-time CGM (rtCGM) and intermittently scanned CGM (isCGM), often referred to as flash glucose monitoring. rtCGM systems, such as the Dexcom G6/G7 or Medtronic Guardian, automatically send glucose data to the receiver or smart device without requiring patient interaction. They often include customizable alarms and alerts for high or low glucose levels, and can feature predictive alerts that warn of impending hypo- or hyperglycemia. isCGM systems, like the FreeStyle Libre series, require the user to actively scan the sensor with a reader or smartphone to obtain the current glucose reading and historical data, typically showing trends over the last 8 hours. Both types provide trend arrows, indicating the direction and rate of glucose change, which is crucial for proactive management decisions regarding food, exercise, and medication dosages.
2.2 Impact on Patient Outcomes
The integration of CGM into T2D management has demonstrated significant improvements in glycemic control, even for individuals not on intensive insulin regimens. A meta-analysis published in The Lancet Diabetes & Endocrinology highlighted that CGM use is associated with a mean reduction in HbA1c of approximately 0.2% to 0.4% in T2D patients, which, while seemingly modest, translates to a clinically meaningful decrease in the risk of diabetes-related complications over time. Beyond HbA1c, CGMs offer superior insights into glucose variability – the degree of fluctuation in glucose levels – which is an independent risk factor for complications and often missed by HbA1c alone. By providing continuous data, CGMs enable healthcare providers and patients to identify patterns of hyperglycemia (e.g., post-meal spikes, dawn phenomenon) and hypoglycemia (e.g., nocturnal lows) that were previously undetectable.
Crucially, CGM systems facilitate a greater ‘Time In Range’ (TIR), defined as the percentage of time spent within the optimal glucose range (typically 70-180 mg/dL or 3.9-10.0 mmol/L). Increased TIR has emerged as a key metric correlating with reduced risk of microvascular complications (Battelino et al., 2019). Studies, including the pivotal trial involving the Omnipod® 5 AID System presented at the American Diabetes Association’s 84th Scientific Sessions, have showcased improved glycemic control, particularly through increased TIR and reduced ‘Time Below Range’ (TBR), in adults with T2D, even those not using basal-bolus insulin regimens (diabetes.org). The ability to see immediate feedback on how food choices, exercise, and medication affect glucose levels empowers patients to make informed, real-time adjustments, leading to more proactive and personalized diabetes management.
2.3 Quality of Life and Healthcare Efficiency
The real-time data and actionable insights provided by CGMs significantly enhance the quality of life for individuals with T2D. The most immediate benefit is the drastic reduction or elimination of painful and inconvenient fingerstick testing. This alleviates the physical discomfort and the mental burden associated with frequent manual checks. Furthermore, the alerts for impending hypoglycemia or hyperglycemia provide a sense of security, particularly during sleep, reducing the ‘fear of hypoglycemia’ which can be a debilitating psychological aspect of diabetes management. Patients report improved sleep quality, increased confidence in managing their condition, and greater flexibility in their daily routines, including exercise and social activities.
From a healthcare efficiency perspective, CGMs enable a more streamlined and data-driven approach to clinical care. The comprehensive glucose data generated can be securely shared with healthcare providers remotely, facilitating virtual consultations and reducing the need for frequent in-person visits. This remote monitoring capability allows clinicians to identify trends, adjust medication dosages (e.g., insulin, sulfonylureas), and provide targeted advice more efficiently. For instance, a detailed 14-day CGM report can offer far more actionable information than a single HbA1c test or scattered fingerstick readings, leading to more precise and timely therapeutic adjustments. This proactive management can potentially reduce emergency room visits and hospitalizations related to acute glycemic events, thereby contributing to overall healthcare cost savings.
2.4 Challenges and Considerations
Despite their undeniable advantages, the widespread adoption and optimal utilization of CGM systems face several challenges. A primary concern is cost, which remains a significant barrier for many patients globally, especially when insurance coverage is limited or non-existent. The recurring expense of sensors, which need regular replacement, adds to the long-term financial burden. Technical considerations include sensor accuracy, which can vary between devices and at different glucose ranges (e.g., lower accuracy during rapid glucose changes or at very low/high levels), and the ‘lag time’ between blood glucose and interstitial fluid glucose readings. While modern CGMs have improved accuracy, represented by Mean Absolute Relative Difference (MARD) values typically under 10%, these factors still need to be understood by users.
Patients may also experience skin irritation, adhesive allergies, or discomfort at the sensor insertion site. Data interpretation can be complex; while trend arrows are intuitive, understanding the nuanced interplay of diet, exercise, stress, and medication on glucose profiles requires education. Healthcare providers, in turn, require training to effectively interpret the voluminous data generated by CGMs and integrate it into their clinical decision-making processes, often necessitating specialized software and standardized reporting formats. Furthermore, cybersecurity of data transmission and storage, ensuring patient privacy, is a critical ongoing concern. Finally, the regulatory landscape for CGM devices, including approval processes and guidelines for use in different T2D populations (e.g., insulin-treated vs. non-insulin-treated), continues to evolve.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
3. Automated Insulin Delivery (AID) Systems
3.1 Overview of AID Technology
Automated Insulin Delivery (AID) systems, often colloquially referred to as ‘artificial pancreas’ systems, represent the pinnacle of current diabetes technology, integrating the capabilities of continuous glucose monitoring with smart insulin pumps through sophisticated control algorithms. The overarching goal of AID systems is to automate the delivery of insulin to approximate the glucose-regulating function of a healthy pancreas, thereby minimizing the need for manual intervention and reducing the burden of diabetes management. A typical AID system comprises three core components: a CGM device that continuously measures glucose levels, an insulin pump that delivers insulin subcutaneously, and a control algorithm (often housed in the pump itself or a paired smartphone) that acts as the ‘brain,’ analyzing CGM data and instructing the pump on insulin delivery.
These systems operate on a closed-loop principle, meaning the algorithm continuously adjusts insulin delivery based on real-time and often predictive glucose readings. The control algorithms vary in complexity and sophistication, ranging from proportional-integral-derivative (PID) controllers to model predictive control (MPC) algorithms. MPC algorithms are particularly advanced, using mathematical models of glucose metabolism, insulin pharmacokinetics, and anticipated future glucose levels to predict glucose trajectories and adjust insulin delivery preemptively. Most commercially available AID systems for T2D are currently ‘hybrid closed-loop’ systems, meaning they automate basal insulin delivery and make adjustments to bolus insulin, but still require the user to manually input mealtime carbohydrates and confirm bolus doses. Examples include the Medtronic MiniMed 780G and the Tandem Control-IQ, which are predominantly used in Type 1 Diabetes but are increasingly being explored and adopted for insulin-dependent Type 2 Diabetes, as well as the Omnipod 5, which received specific indications for T2D in insulin-requiring adults (en.wikipedia.org).
3.2 Impact on Patient Outcomes
Clinical trials and real-world evidence have consistently demonstrated that AID systems can significantly improve glycemic control in T2D patients requiring insulin, leading to substantial reductions in HbA1c levels. For example, studies have shown reductions in HbA1c ranging from 0.5% to 1.0% or more, depending on baseline control. Beyond HbA1c, AID systems excel at minimizing glucose variability and increasing Time In Range (TIR), as they are constantly working to keep glucose levels within the target range. A study published in Diabetes, Obesity & Metabolism highlighted that AID systems effectively manage blood glucose levels in T2D patients, enhancing overall diabetes management, particularly by reducing hypoglycemia and improving TIR (pubmed.ncbi.nlm.nih.gov).
One of the most profound impacts of AID systems is the significant reduction in the incidence and severity of hypoglycemic events, especially nocturnal hypoglycemia. By suspending or reducing insulin delivery when glucose levels are predicted to fall, or when they drop below a certain threshold, AID systems provide a crucial safety net. This is particularly beneficial for T2D patients, who may be at risk for severe hypoglycemia with complex insulin regimens. Conversely, these systems can also deliver micro-boluses or increase basal rates to mitigate post-meal hyperglycemia, flattening glucose curves and reducing time spent in hyperglycemic states, which contributes to long-term complication risk. The continuous, adaptive nature of insulin delivery by AID systems leads to more stable glucose profiles, thereby mitigating glucose excursions and the physiological stress associated with them.
3.3 Quality of Life and Healthcare Efficiency
The automation offered by AID systems significantly alleviates the immense ‘cognitive load’ associated with daily diabetes management. Patients no longer need to constantly monitor glucose, calculate complex insulin doses, or manually adjust basal rates based on exercise, meals, or stress. This reduction in decision-making burden leads to a substantial improvement in quality of life, fostering greater freedom, spontaneity, and peace of mind. Users often report improved sleep quality due to reduced worry about nocturnal glucose fluctuations and fewer disruptive alarms. This enhanced mental well-being contributes to greater overall satisfaction and reduces the psychological distress often associated with chronic disease management.
In terms of healthcare efficiency, AID systems have the potential to reduce the frequency of acute diabetes-related complications requiring urgent care or hospitalization. By maintaining tighter and more stable glycemic control, they can decrease the incidence of severe hypoglycemia and diabetic ketoacidosis (DKA), both of which are costly and resource-intensive events. The wealth of data collected by AID systems, including insulin delivery logs and glucose trends, provides clinicians with invaluable information for fine-tuning treatment plans during follow-up appointments. This data-driven approach allows for more efficient consultations, focused on proactive adjustments rather than reactive crisis management, thereby optimizing the allocation of healthcare resources and potentially lowering long-term healthcare costs associated with chronic complications.
3.4 Challenges and Considerations
Despite their revolutionary potential, the widespread adoption of AID systems in T2D management faces several significant hurdles. Device affordability and insurance coverage remain major barriers globally. The upfront cost of the pump and recurring expenses for insulin pump supplies (infusion sets, reservoirs) and CGM sensors can be prohibitive. Regulatory bodies, such as the FDA, have specific pathways for AID systems, ensuring their safety and efficacy, but the complexity of these devices necessitates ongoing post-market surveillance and reporting.
Patient education and comprehensive training are paramount for successful implementation. While AID systems automate much of the decision-making, patients still need to understand how to operate the system, troubleshoot issues, respond to alarms, and manage specific scenarios like mealtime bolusing, exercise, and sick days. There is a learning curve, and inadequate training can lead to frustration or suboptimal outcomes. Cybersecurity is another critical concern, as these interconnected medical devices handle sensitive health data and are vulnerable to potential breaches or malfunctions that could compromise patient safety. Furthermore, the variability in individual responses to insulin and metabolic profiles necessitates personalized calibration and ongoing clinical supervision to optimize the system’s performance for each patient. Algorithm ‘aggressiveness’ and responsiveness may also need careful titration, especially in T2D populations who may have greater insulin resistance and different metabolic kinetics compared to T1D patients. Finally, the challenge of integrating data from AID systems into electronic health records (EHRs) for a holistic view of patient health also persists, requiring robust interoperability solutions.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
4. Smart Insulin Pens
4.1 Overview of Smart Insulin Pens
Smart insulin pens represent a significant technological evolution from traditional insulin pens, designed to address common challenges in insulin therapy, particularly adherence and accurate dose tracking. These devices build upon the convenience of pen injectors by incorporating digital functionalities that enhance data capture and inform decision-making. At their core, smart insulin pens record the date, time, and dosage of each insulin injection. Many models feature Bluetooth connectivity, allowing this data to be wirelessly transmitted to a compatible smartphone application or a digital health platform.
Beyond basic dose logging, advanced smart pens integrate additional functionalities. Some models include a built-in bolus calculator, which, when linked with a CGM or manually inputted blood glucose levels and carbohydrate counts, can recommend appropriate mealtime or correction insulin doses. This feature helps to minimize dosing errors and alleviate the mental burden of complex calculations for patients. Other features may include dose reminders, ‘last dose’ memory (preventing accidental double dosing), temperature monitoring (ensuring insulin efficacy), and sharing capabilities that allow patients to transmit their dosing history directly to their healthcare providers. Examples include the InPen (acquired by Medtronic), NovoPen® 6 and NovoPen Echo® Plus (Novo Nordisk), and HumaPen Savvio (Eli Lilly), each offering a slightly different suite of features and integration capabilities with various apps and CGMs. These pens serve as a bridge, offering some of the data-driven benefits of pumps without the full commitment or complexity of continuous infusion.
4.2 Impact on Patient Outcomes
The use of smart insulin pens has been demonstrably associated with improved adherence to insulin therapy, a critical factor in achieving and maintaining glycemic control. By providing an automatic, accurate record of every injection, these pens help patients overcome challenges such as forgetting if a dose was taken or remembering the exact timing and amount. This detailed logging allows patients to reflect on their patterns and healthcare providers to pinpoint specific adherence issues. A study published in the Journal of Diabetes Science and Technology found that smart insulin pens can significantly enhance insulin dosing accuracy and reduce the risk of dosing errors by providing real-time feedback and calculation assistance (pubmed.ncbi.nlm.nih.gov).
Improved adherence and dosing accuracy directly translate to better glycemic control, evidenced by reductions in HbA1c levels and a decreased incidence of both hypoglycemic and hyperglycemic events. The ability of the pens to provide detailed insulin delivery logs, often integrated with glucose data from CGMs, allows for more precise and individualized therapy adjustments. Clinicians can identify correlations between insulin doses, meal timing, and glucose responses, enabling them to fine-tune insulin regimens more effectively. This data-driven approach fosters a better understanding of individual insulin needs and responses, empowering both patients and providers to optimize therapy and mitigate glucose excursions.
4.3 Quality of Life and Healthcare Efficiency
Smart insulin pens significantly simplify the insulin administration process, making it more convenient and less stressful for patients. The automation of dose logging frees patients from the tedious task of manual record-keeping, which can be prone to errors and often neglected. The ‘last dose’ memory feature provides reassurance and prevents accidental double-dosing, a common source of anxiety and potential hypoglycemia. This enhanced convenience and sense of security contribute to an improved quality of life, allowing patients to manage their diabetes with greater confidence and less intrusion into their daily lives.
From a healthcare efficiency standpoint, smart insulin pens facilitate a more collaborative and informed patient-provider relationship. The seamless data sharing capabilities enable healthcare providers to access comprehensive insulin dosing patterns, eliminating the reliance on subjective patient recall or incomplete manual logs. During clinic visits, this readily available, objective data allows for more productive discussions, enabling clinicians to make faster, more accurate treatment decisions and personalize care plans with greater precision. This can reduce the time spent deciphering incomplete records and lead to more effective therapy adjustments, potentially decreasing the need for follow-up visits and minimizing the risk of complications that require intensive medical intervention.
4.4 Challenges and Considerations
Despite their benefits, the widespread adoption of smart insulin pens encounters several challenges. The initial cost of the smart pen devices, while generally less than an insulin pump, can still be a barrier, particularly if not fully covered by insurance. The ongoing need for connectivity (e.g., Bluetooth pairing) and battery maintenance (recharging or replacing batteries) introduces additional steps and potential technical issues that some users may find cumbersome. Connectivity problems, such as dropped signals or compatibility issues with certain smartphone models or operating systems, can disrupt data flow and lead to frustration.
Moreover, there can be a learning curve associated with effectively using these devices, particularly for patients who are less technologically literate. Comprehensive patient education is essential to ensure they understand how to use the pen’s features, interpret the data presented in the accompanying app, and integrate this information into their self-management routine. Data privacy and security are also important considerations, as insulin dosing data is highly sensitive health information. Manufacturers and app developers must adhere to strict data protection regulations (e.g., GDPR, HIPAA) to safeguard patient information. Finally, the lack of universal interoperability standards can make it challenging to integrate data from various smart pens with different digital health platforms or electronic health records, potentially creating data silos rather than a unified view of patient care.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
5. Digital Health Platforms
5.1 Overview of Digital Health Platforms
Digital health platforms represent a broad and rapidly expanding category of technological interventions designed to support and enhance diabetes management. These platforms leverage ubiquitous digital tools – from mobile applications to advanced telehealth infrastructure – to provide comprehensive support, education, and connectivity. Their scope is vast, encompassing a range of functionalities that empower patients in self-management and facilitate more efficient clinical care.
Key components of digital health platforms include:
* Mobile Applications (Apps): These are often the most visible aspect, offering functionalities such as glucose tracking (manual input or integration with CGMs/smart pens), medication reminders, food logging (calorie counting, carbohydrate estimation), activity tracking (often linking with wearables), educational content (e.g., about nutrition, exercise, medication), and goal setting. Many apps provide visual feedback on trends and patterns, helping users understand the impact of their lifestyle choices. Examples include Glooko, One Drop, Livongo, and proprietary apps from CGM/AID manufacturers.
* Telehealth Services: These involve virtual consultations with healthcare providers (doctors, nurses, dietitians, diabetes educators) via video, phone, or secure messaging. Telehealth reduces geographical barriers, improves access to specialists, and allows for more frequent check-ins, moving towards a continuous care model rather than episodic visits.
* Online Portals and Web-based Platforms: These often serve as central hubs for patient records, educational resources, community forums for peer support, and secure communication channels with care teams. They can also facilitate prescription refills and appointment scheduling.
* Wearable Integration: Beyond CGMs, many platforms integrate data from other wearables, such as smartwatches or fitness trackers, to provide a more holistic view of activity levels, sleep patterns, and heart rate, all of which can influence glucose management.
* Digital Therapeutics (DTx): This emerging subset refers to evidence-based therapeutic interventions delivered by software programs to prevent, manage, or treat a medical disorder or disease. DTx for T2D might involve highly structured, personalized programs designed to induce specific behavioral changes (e.g., weight loss, increased physical activity) through tailored feedback and coaching, often leveraging AI.
5.2 Impact on Patient Outcomes
Digital health platforms have been robustly demonstrated to improve self-management behaviors and, consequently, glycemic control in T2D patients. By providing accessible tools for tracking key metrics (glucose, diet, activity, medication adherence), these platforms foster greater patient engagement and accountability. The immediate feedback loop helps patients connect their actions with their physiological responses, leading to more informed decision-making and sustainable behavioral changes. For instance, a study published in Diabetes Technology and Therapeutics demonstrated that CGM significantly enhances glycemic control in adults with T2D who are not using insulin, particularly when coupled with digital health platforms that provide interpretative guidance (diabetes.org).
Meta-analyses consistently show that digital health interventions, including mobile apps and telehealth, are associated with modest but clinically significant reductions in HbA1c, typically ranging from 0.3% to 0.8%. Beyond glycemic metrics, these platforms can lead to improvements in other crucial health outcomes such as weight loss, increased physical activity levels, and better medication adherence. The educational components within many platforms enhance patient literacy about diabetes, empowering them to take a more active role in their care. Peer support forums can also provide psychological benefits, reducing feelings of isolation and increasing motivation through shared experiences.
5.3 Quality of Life and Healthcare Efficiency
Digital health platforms significantly enhance the quality of life for T2D patients by offering unparalleled flexibility, accessibility, and convenience in managing their condition. Patients can access critical information, log data, and communicate with their care team from virtually anywhere, reducing the need for time-consuming and often disruptive in-person clinic visits. This empowerment fosters a greater sense of control over their health, alleviating some of the mental burden of chronic disease management. Personalized feedback and educational resources delivered through these platforms can increase patient confidence and self-efficacy.
From a healthcare efficiency perspective, digital health platforms are transformative. They can drastically reduce the operational burden on clinics by shifting certain aspects of care to remote settings. Telehealth, in particular, has proven invaluable in reducing travel time and costs for patients and providers, expanding access to specialist care for those in remote areas, and enabling more frequent, shorter interactions that can be more proactive than traditional episodic visits. These platforms can also facilitate population health management by allowing healthcare systems to monitor groups of patients, identify those at higher risk, and intervene proactively. The continuous flow of data can lead to more timely adjustments to treatment plans, potentially preventing costly acute complications and reducing hospitalizations, thereby optimizing resource allocation and driving overall cost savings within the healthcare system.
5.4 Challenges and Considerations
Despite their immense potential, the widespread and equitable adoption of digital health platforms faces several significant barriers. Technological literacy remains a crucial challenge; older adults or individuals from lower socioeconomic backgrounds may lack the necessary skills or confidence to navigate complex apps or telehealth interfaces. This contributes to a digital divide, exacerbating health disparities if access to technology, reliable internet, and digital literacy training is not universal. Furthermore, data privacy and security concerns are paramount. Patients need assurance that their highly sensitive health information is protected from breaches and misuse. Developers and healthcare providers must adhere strictly to regulations like HIPAA in the United States or GDPR in Europe, implementing robust encryption and access controls.
Interoperability with existing electronic health record (EHR) systems is another major hurdle. Many digital health apps operate in silos, making it challenging for clinicians to integrate data seamlessly into patient charts for a holistic view of care. Lack of standardized data formats and communication protocols hinders comprehensive data aggregation. The regulatory landscape for digital health tools is also evolving, with questions around the clinical validation, safety, and efficacy of certain apps and digital therapeutics requiring clear guidelines. User engagement and retention are also critical; many health apps see high rates of abandonment after initial use. Designing platforms that are intuitive, engaging, and provide continuous value is key to long-term adherence. Finally, reimbursement models for telehealth and digital therapeutics are still developing, which can impede adoption by healthcare providers and limit patient access if services are not covered by insurance.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
6. Emerging Technologies
The landscape of diabetes technology is characterized by relentless innovation, with several groundbreaking technologies currently in various stages of research, development, and early clinical trials. These emerging solutions promise to further revolutionize T2D management, offering unprecedented levels of convenience, precision, and personalization.
6.1 Non-Invasive Glucose Monitoring
The holy grail of diabetes technology has long been the development of truly non-invasive glucose monitoring, eliminating the need for any skin puncture. While this field has seen numerous attempts and challenges, research continues with promising avenues. Various techniques are under investigation, broadly categorized by their measurement principles:
- Optical Methods: These techniques utilize light to analyze glucose levels.
- Raman Spectroscopy: Involves shining a laser onto the skin and analyzing the scattered light, which produces a unique spectral ‘fingerprint’ based on molecular vibrations, including those of glucose. The challenge lies in isolating the glucose signal from other skin components and achieving sufficient sensitivity and specificity.
- Near-Infrared (NIR) Spectroscopy: Uses NIR light, which penetrates tissue, to detect changes in absorption patterns influenced by glucose concentration. Issues include interference from skin pigmentation, temperature, and other analytes.
- Photoacoustic Spectroscopy: Employs laser pulses to induce sound waves in tissue, which are then detected and correlated with glucose levels. This method shows promise for deeper tissue penetration.
- Electromagnetic Methods:
- Radiofrequency/Microwave Technology: Uses low-power electromagnetic waves to measure dielectric properties of tissue, which change with glucose concentration.
- Biochemical Methods:
- Tear Fluid/Sweat Analysis: Investigating glucose levels in tear fluid or sweat using contact lenses or skin patches. While promising for convenience, correlation with blood glucose can be inconsistent due to lag time and varying concentrations.
Companies like K’Watch and Cnoga Medical are pursuing varying optical or electromagnetic approaches, though none have yet achieved the clinical accuracy and regulatory approval comparable to invasive CGMs for widespread primary use. The technical hurdles are immense, including achieving consistent accuracy across diverse populations, managing interference from environmental factors and biological variability, and ensuring robustness for daily wear. While a truly reliable non-invasive CGM remains elusive, continued advancements in sensor technology and signal processing offer hope for future breakthroughs (en.wikipedia.org).
6.2 AI-Driven Predictive Analytics
Artificial Intelligence (AI) and machine learning (ML) algorithms are rapidly transforming predictive analytics in diabetes management, moving beyond simple trend analysis to sophisticated, real-time forecasting and personalized recommendations. These systems leverage vast datasets – including CGM readings, insulin pump data, dietary intake, physical activity levels, sleep patterns, stress indicators, and even environmental factors – to build intricate models of individual glucose metabolism.
Applications of AI in T2D management include:
- Real-time Glucose Prediction: Advanced ML models can predict future glucose levels (e.g., 30-60 minutes ahead) with high accuracy, enabling proactive interventions to avert hypoglycemia or hyperglycemia. This allows patients to adjust food intake or activity, or for AID systems to modify insulin delivery, before glucose excursions occur.
- Personalized Insulin Dosing Optimization: AI algorithms can learn an individual’s unique response to insulin, carbohydrates, and exercise. This allows for highly personalized recommendations for mealtime boluses, correction doses, and basal rate adjustments, optimizing therapy beyond standardized guidelines. Reinforcement learning (RL) models, for instance, can dynamically learn optimal basal-bolus strategies (A Dual Mode Adaptive Basal-Bolus Advisor Based on Reinforcement Learning, 2019 arxiv.org).
- Identification of High-Risk Patients: AI can analyze vast amounts of health record data to identify T2D patients at higher risk of developing complications (e.g., retinopathy, nephropathy, cardiovascular disease) or experiencing acute events, allowing for early intervention and targeted preventive care.
- Clinical Decision Support: AI-powered tools can assist healthcare providers by flagging concerning trends, suggesting therapy adjustments, and providing evidence-based recommendations, particularly for complex cases (Nurse-in-the-Loop Artificial Intelligence for Precision Management of Type 2 Diabetes in a Clinical Trial Utilizing Transfer-Learned Predictive Digital Twin, 2024 arxiv.org).
The power of AI lies in its ability to identify subtle patterns and make nuanced decisions that human clinicians or simpler algorithms might miss, leading to truly individualized and proactive diabetes care. However, challenges include the need for high-quality, diverse datasets for training, ensuring algorithmic fairness and avoiding bias, and establishing clear regulatory pathways for AI-driven medical devices.
6.3 Personalized Feedback Systems
Personalized feedback systems leverage data collected from various devices (CGM, wearables, smart pens) and often integrate AI analytics to provide tailored, actionable advice and support to patients. These systems move beyond generic health tips to deliver highly individualized guidance based on a patient’s specific glucose patterns, behaviors, and goals. The goal is to promote sustained behavior change and improved self-management.
Key characteristics include:
- Contextualized Insights: Instead of merely presenting raw data, these systems interpret it. For example, a system might not just show a glucose spike but also correlate it with a specific meal logged by the patient and suggest alternative food choices or exercise timings for future similar scenarios.
- Behavioral Nudging: Drawing from behavioral science and psychology, these systems can provide ‘nudges’ or motivational messages to encourage desired actions, such as ‘You’ve been less active today, consider a short walk to help manage your glucose,’ or ‘Remember your medication is due in 30 minutes.’ Reinforcement learning systems can be designed to encourage physical activity, adapting feedback based on user responses (A Reinforcement Learning System to Encourage Physical Activity in Diabetes Patients, 2016 arxiv.org).
- Adaptive Learning: The systems learn from patient responses and preferences over time, continually refining their feedback to be more effective and relevant. For example, if a patient responds well to positive reinforcement but not to direct commands, the system can adapt its communication style.
- Virtual Coaching: Some platforms incorporate AI-powered virtual coaches that provide ongoing support, answer questions, and guide patients through educational modules or goal-setting exercises, offering a scalable solution for personalized support.
These systems aim to bridge the gap between data and action, empowering patients to make better daily decisions and fostering long-term adherence to healthy habits, thereby improving overall glycemic control and quality of life.
6.4 Smart Insulin Patches and Other Innovations
Beyond the technologies discussed, the innovation pipeline includes other exciting developments:
- Smart Insulin Patches: These discreet, wearable patches are being developed to deliver insulin automatically and painlessly through microneedles or other transdermal methods. Some prototypes are ‘glucose-responsive,’ meaning they release insulin only when glucose levels are high, aiming for a truly autonomous and smart insulin delivery system without external control (e.g., en.wikipedia.org). While still largely in research, these hold the promise of simplifying insulin therapy significantly.
- Dual-Hormone AID Systems: Expanding beyond insulin, some AID systems are exploring the co-delivery of glucagon (or other glucose-raising hormones) alongside insulin to prevent hypoglycemia more effectively, offering even tighter and safer glucose control, particularly for T1D, but with potential future applications for complex T2D.
- Continuous Ketone Monitoring: While primarily for T1D, continuous ketone monitoring could also benefit T2D patients at risk of ketoacidosis, providing early warnings and guiding intervention.
These emerging technologies underscore a future where diabetes management is not only highly personalized and data-driven but also increasingly integrated, automated, and less burdensome for the individual.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
7. Impact Assessment
The integration of technological innovations into Type 2 Diabetes management has brought about a paradigm shift, yielding significant and measurable impacts across several critical domains.
7.1 Patient Outcomes
The most profound impact of these technologies is the consistent improvement in glycemic control. CGM systems provide granular data that reveals previously unseen glucose patterns, leading to more targeted interventions and significant reductions in HbA1c. AID systems further refine this, demonstrating substantial decreases in HbA1c, increased Time In Range (TIR), and critically, a marked reduction in the incidence and severity of hypoglycemic events, especially nocturnal hypoglycemia, which is a major concern for insulin-treated T2D patients. Smart insulin pens enhance adherence and dosing accuracy, directly contributing to better glycemic stability. Digital health platforms, through improved self-management and education, empower patients to make better daily choices, resulting in better overall glucose control and the mitigation of acute glycemic excursions. Collectively, these technologies contribute to a more stable and optimized metabolic state, which is foundational to reducing the long-term risk of both microvascular (retinopathy, nephropathy, neuropathy) and macrovascular complications (cardiovascular disease, stroke) associated with chronic hyperglycemia and glucose variability.
7.2 Quality of Life
Beyond clinical metrics, these innovations have a transformative effect on the quality of life for individuals with T2D. The reduction in daily disease burden is immense. CGMs minimize or eliminate painful fingersticks, AID systems automate complex decision-making, and smart pens simplify dose tracking. This ‘cognitive offloading’ frees mental energy, reducing stress, anxiety, and the ever-present fear of hypoglycemia. Patients report greater flexibility in their lifestyle, improved sleep quality, increased confidence in managing their condition, and a renewed sense of empowerment and control over their health. The ability to access real-time data and receive personalized feedback fosters a more proactive and less reactive approach to daily life, allowing individuals to integrate diabetes management more seamlessly into their routines and live fuller, more unrestricted lives.
7.3 Healthcare Efficiency
From an operational and economic perspective, these technologies offer considerable potential for enhancing healthcare efficiency and resource optimization. Remote monitoring capabilities, facilitated by CGMs and digital health platforms, allow for more frequent and proactive clinical oversight, potentially reducing the need for costly in-person visits. Telehealth services expand access to specialized care, particularly for underserved or geographically isolated populations, while also reducing patient travel time and associated costs. By enabling better glycemic control and reducing acute complications (severe hypoglycemia, DKA), these technologies can significantly decrease emergency room visits and hospitalizations, leading to substantial cost savings for healthcare systems. The wealth of objective data collected streamlines clinic appointments, allowing healthcare providers to make more informed and efficient treatment adjustments, shifting the focus from reactive problem-solving to proactive, preventative care. This also supports population health management initiatives by identifying and intervening with high-risk individuals more effectively.
7.4 Accessibility
While the promise of these innovations is vast, ensuring equitable access remains a critical challenge. Disparities in access are multifaceted, often driven by:
- Affordability: The high upfront and recurring costs of devices (CGMs, AID systems, smart pens) and associated supplies can be prohibitive for many, especially in regions with limited insurance coverage or lower socioeconomic status.
- Insurance Coverage: Coverage policies vary widely by country, region, and individual plan, often creating a postcode lottery for advanced diabetes technologies.
- Digital Literacy: A significant portion of the T2D population, particularly older adults, may lack the technological proficiency or confidence to effectively use apps, interpret data, or engage in telehealth, creating a ‘digital divide.’
- Infrastructure: Reliable internet access and smartphone ownership are prerequisites for many of these technologies, which are not universally available, particularly in rural or low-resource settings.
- Cultural Competence: Digital health tools and educational materials must be culturally sensitive and linguistically appropriate to resonate with diverse patient populations.
Addressing these issues requires a concerted effort involving policymakers, healthcare providers, technology developers, and patient advocacy groups to implement supportive policies, expand insurance coverage, provide digital literacy training, and design user-friendly, inclusive technologies to ensure these transformative benefits are accessible to all who can benefit.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
8. Challenges and Future Directions
The rapid evolution and integration of diabetes technologies, while profoundly beneficial, concurrently introduce a new set of complex challenges that demand proactive strategies and collaborative solutions.
8.1 Data Interpretation and Integration
The sheer volume and complexity of data generated by CGMs, AID systems, smart pens, and digital health platforms present a significant challenge. Clinicians, already burdened by heavy workloads, must now navigate and interpret intricate glucose profiles, insulin delivery patterns, and behavioral data. This ‘data deluge’ requires sophisticated analytical tools and expertise to extract actionable insights.
Future Directions:
* Standardized Data Formats: Development and mandatory adoption of universal data exchange standards (e.g., FHIR – Fast Healthcare Interoperability Resources) are crucial to enable seamless interoperability between devices, apps, and electronic health records (EHRs).
* Clinical Decision Support Systems (CDSS): AI-powered CDSS can process vast datasets, identify trends, flag anomalies, and provide evidence-based recommendations to clinicians, reducing cognitive load and enhancing diagnostic and therapeutic accuracy.
* Training for Clinicians: Comprehensive training programs are needed to equip healthcare professionals with the skills to interpret complex CGM and AID data, understand the nuances of various algorithms, and effectively integrate technological insights into personalized care plans. This necessitates a new field of ‘diabetic informatics.’
* Patient-Friendly Reports: Developers must continue to refine user interfaces and reporting tools to make data digestible and actionable for both patients and clinicians.
8.2 Regulatory and Ethical Considerations
The rapid pace of technological innovation often outstrips the development of regulatory frameworks. Ensuring the safety, efficacy, and ethical deployment of these advanced medical devices and software-as-a-medical-device (SaMD) is paramount.
Future Directions:
* Adaptive Regulatory Pathways: Regulatory bodies (e.g., FDA, EMA) need to establish agile and adaptive approval pathways that can keep pace with continuous innovation, particularly for AI-driven algorithms that learn and evolve over time, while maintaining stringent safety standards.
* Data Governance and Privacy: Robust frameworks are required to govern the collection, storage, sharing, and use of highly sensitive health data, ensuring patient consent, anonymization where appropriate, and strict adherence to privacy regulations (e.g., GDPR, HIPAA).
* Algorithmic Bias: As AI plays a greater role, ethical considerations around algorithmic bias (e.g., if AI models are trained on unrepresentative datasets) must be addressed to ensure equitable outcomes across diverse patient populations.
* Liability: Clear guidelines on liability in the event of device malfunction or AI-driven errors are necessary to protect both patients and providers.
8.3 Patient Education and Support
The full benefits of these technologies can only be realized if patients are adequately educated and supported in their use. Lack of understanding can lead to suboptimal outcomes, frustration, and device abandonment.
Future Directions:
* Comprehensive, Tailored Education: Develop personalized education programs that consider individual patient literacy levels, learning styles, and cultural backgrounds. This should include initial training, ongoing support, and troubleshooting resources.
* Role of Diabetes Educators: Expand the role and capacity of certified diabetes care and education specialists (CDCES) to provide specialized training and support for advanced technologies.
* Peer Support Networks: Facilitate and support peer communities (online and offline) where patients can share experiences, tips, and encouragement, which has been shown to improve adherence and outcomes.
* Digital Skills Training: Implement community-based programs to improve digital literacy among underserved populations, helping bridge the digital divide.
8.4 Cybersecurity
As medical devices become increasingly connected, they become potential targets for cyberattacks, posing risks to patient privacy and safety.
Future Directions:
* Robust Security Protocols: Implement industry-leading encryption, authentication, and intrusion detection systems for all connected devices and platforms.
* Regular Audits and Updates: Manufacturers must conduct regular security audits and provide timely software updates to address newly identified vulnerabilities.
* Incident Response Plans: Healthcare organizations and device manufacturers need clear, rehearsed incident response plans for data breaches or device compromises.
8.5 Reimbursement Models
The high cost of many advanced diabetes technologies remains a significant barrier to equitable access.
Future Directions:
* Value-Based Reimbursement: Develop reimbursement models that recognize the long-term value of these technologies in preventing complications and improving quality of life, rather than focusing solely on upfront costs.
* Subsidies and Access Programs: Governments and health systems should explore subsidies or specific access programs for vulnerable populations to ensure equitable distribution.
* Evidence Generation: Continuous generation of real-world evidence on the cost-effectiveness and long-term benefits of these technologies is crucial for advocating for broader insurance coverage.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
9. Conclusion
The journey of Type 2 diabetes management has been profoundly transformed by an accelerating wave of technological innovations. Continuous Glucose Monitoring systems, Automated Insulin Delivery systems, smart insulin pens, and diverse digital health platforms are no longer aspirational concepts but tangible realities that are significantly enhancing glycemic control, reducing acute complications, and substantially improving the quality of life for millions living with T2D. These tools empower patients with unprecedented insight and control, while simultaneously providing healthcare providers with the data necessary for truly personalized and proactive care, thereby streamlining healthcare delivery and fostering efficiency.
Yet, the full realization of this transformative potential hinges on effectively navigating a complex landscape of challenges. The sheer volume and intricate nature of generated data necessitate advanced interpretative tools and specialized clinical expertise. Ethical and regulatory frameworks must evolve dynamically to ensure the safety, privacy, and equitable deployment of these rapidly developing technologies. Crucially, addressing disparities in accessibility – driven by cost, insurance coverage, digital literacy, and infrastructure limitations – is paramount to ensure that these innovations do not exacerbate existing health inequities. Comprehensive patient education and ongoing support are fundamental to fostering effective engagement and maximizing benefits.
Looking ahead, emerging technologies such as non-invasive glucose monitoring and sophisticated AI-driven predictive analytics promise even greater strides towards a future where diabetes management is not only highly precise and data-informed but also increasingly seamless, autonomous, and integrated into daily life. Achieving this vision will require sustained, collaborative efforts from patients, healthcare providers, technology developers, policymakers, and researchers. By collectively addressing the current hurdles and strategically investing in future directions, we can unlock the full promise of technology to fundamentally improve the lives of all individuals affected by Type 2 diabetes, moving closer to a future where effective management is a universal standard, not a privileged exception.
Many thanks to our sponsor Esdebe who helped us prepare this research report.
References
-
American Diabetes Association. (2024). Breakthrough Studies on Automated Insulin Delivery and CGM for Type 2 Diabetes Unveiled at ADA Scientific Sessions. Retrieved from https://diabetes.org/newsroom/press-releases/breakthrough-studies-automated-insulin-delivery-and-cgm-type-2-diabetes
-
Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control. (2024). PubMed. Retrieved from https://pubmed.ncbi.nlm.nih.gov/36635028/
-
Noninvasive Glucose Monitor. (n.d.). In Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Noninvasive_glucose_monitor
-
Automated Insulin Delivery System. (n.d.). In Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Automated_insulin_delivery_system
-
Smart Insulin Patch. (n.d.). In Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Smart_insulin_patch
-
A Reinforcement Learning System to Encourage Physical Activity in Diabetes Patients. (2016). arXiv. Retrieved from https://arxiv.org/abs/1605.04070
-
Nurse-in-the-Loop Artificial Intelligence for Precision Management of Type 2 Diabetes in a Clinical Trial Utilizing Transfer-Learned Predictive Digital Twin. (2024). arXiv. Retrieved from https://arxiv.org/abs/2401.02661
-
A Dual Mode Adaptive Basal-Bolus Advisor Based on Reinforcement Learning. (2019). arXiv. Retrieved from https://arxiv.org/abs/1901.01816
-
Battelino, T., Danne, T., Bergenstal, R. M., et al. (2019). Clinical Targets for Continuous Glucose Monitoring-Data Interpretation and Utilization Endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care, 42(8), 1593–1603.
-
IDF Diabetes Atlas. (10th ed.). International Diabetes Federation, Brussels, Belgium. (2021). Retrieved from https://diabetesatlas.org/
-
Long, D., & Fox, G. (2021). Continuous Glucose Monitoring in Type 2 Diabetes: A Review of Clinical Efficacy and Impact on Health Outcomes. The Lancet Diabetes & Endocrinology, 9(7), 446-455.


The potential of AI-driven predictive analytics to personalize insulin dosing is fascinating. How might these algorithms account for the highly variable effects of stress and sleep on an individual’s glucose levels?
That’s a great question! The algorithms could incorporate data from wearable devices that track sleep patterns and stress levels (e.g., heart rate variability). Combining this with historical glucose data could allow the AI to learn how these factors uniquely impact an individual’s glucose, leading to more accurate personalized predictions and dosing recommendations. It’s an exciting area of development!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The discussion of personalized feedback systems is particularly relevant. How can we ensure these systems are culturally sensitive and adaptable to the diverse needs and beliefs of individuals managing T2D across different communities?
That’s a really important point about cultural sensitivity! Perhaps these systems could incorporate community-specific dietary habits and traditional health practices? Gathering feedback from diverse patient groups during development could be invaluable in ensuring these systems are both effective and respectful. What other approaches could we consider?
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
Non-invasive glucose monitoring sounds like something straight out of science fiction! If they can crack that nut, will finger pricks become just a distant, unpleasant memory? Maybe then I’ll *finally* stop flinching every time I see a lancet!
It really does, doesn’t it? Imagine a world without finger pricks! There’s so much research and development currently focused on non-invasive methods. The potential impact on daily life for people managing diabetes is huge. Let’s hope these technologies become a reality soon!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The discussion about data interpretation is critical. As technology generates increasing amounts of patient data, what are the innovative strategies for educating patients so they can understand and act on their personalized insights?
That’s a fantastic question! We’re exploring interactive dashboards with visual aids that translate complex data into easily understandable formats. Gamification and personalized learning modules also show promise in empowering patients to actively manage their health based on these insights. Community support groups could provide peer learning opportunities.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The discussion around accessibility is vital; innovative financing models and community-based programs could help bridge the digital divide, ensuring that these technologies reach underserved populations and promote health equity.
You’re absolutely right about the need for innovative financing! Exploring options like subscription models or tiered pricing, combined with community-led education initiatives, is key. Partnering with local organizations could also help tailor solutions to specific community needs, boosting adoption and long-term success. This approach would address some of the barriers that people face now.
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The discussion about healthcare efficiency is compelling. Telehealth integration, enabled by these technologies, has the potential to revolutionize access to diabetes specialists, especially in underserved areas. Do you think we’ll see more personalized remote monitoring programs in the future?
Absolutely! The potential for personalized remote monitoring is huge. Imagine AI tailoring advice based on individual data trends, proactively connecting patients with specialists. Continuous data streams will enable early interventions and reduce emergency situations in remote communities. Telehealth will definitely evolve!
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
This report highlights the crucial role of continuous data in improving diabetes management. Further research into the optimal frequency and timing of data feedback to patients could maximize its impact on behavior change and long-term adherence.
Thank you for your insightful comment! I agree that understanding the optimal frequency and timing of data feedback is key. I think real-time coaching integrated within the data streams could improve patient engagement. What models of data feedback have you found most effective in promoting behavior change?
Editor: MedTechNews.Uk
Thank you to our Sponsor Esdebe
The report mentions the challenge of algorithmic bias in AI-driven diabetes management. What methods are being developed to ensure AI algorithms are trained on diverse datasets, and how can we mitigate potential biases in their recommendations?